-
Ketamine (Ketalar):
pharmacokinetics5
-
Metabolism: the liver microsomal enzyme system metabolizes
ketamine (Ketalar) involving hydroxylation and demethylation.
-
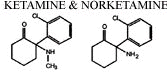
The principal metabolite is norketamine, note below the removal of
the methyl group from ketamine (Ketalar) (left), forming
norketamine.
-
As an aside, the difference between
epinephrine and norepinephrine (Levophed) is that
norepinephrine lacks a methyl group. In the biosynthetic
pathway, epinephrine is formed from norepinephrine (Levophed) by a
methyl transferase enzyme [phenylethanolamine N-methyltransferase].
-
-
Norketamine exhibits about 25%-30% of ketamine (Ketalar).
-
The high lipid solubility of ketamine (Ketalar) results in a
rapid onset of action in a manner similar to that discussed
earlier for other lipid-soluble IV anesthetics, e.g. thiopental (Pentothal).
-
Similarly, recovery from the anesthetic effects is probably due to
redistribution from the brain to other compartments.
-
Time to onset following IV bolus (dosage = 2 mg/kg) is about
30-60 seconds with effect lasting between 10-15 minutes.
-
Volume of distribution (Vd). Recalling that
Vd is determined by measuring plasma drug levels,
it is not surprising that a highly lipid-soluble molecule
would have a very large volume of distribution.
-
Note that clearance is dependent on not only volume
of distribution but also the elimination halftime. CL =
(.693*Vd)/t1/2.
-
By rearranging
the earlier formula, t1/2
= (0.693 · Vd)/CL.
-
For ketamine (Ketalar),
clearance is relatively high at 12-17 ml/kg/minute as a
result of a fairly short elimination halftime (about 2.5
hours).
-
As noted earlier, clearance is mediated
by the liver microsomal enzyme system; therefore, factors
that decrease hepatic blood flow will retard clearance and
prolong ketamine (Ketalar) effect.
-
Ketamine (Ketalar) infusion rate: 30-90 ug/kg/minute, which would be reduced if given in combination with other CNS
depressants.
-
 Ketamine (Ketalar) pharmacology: a summary of organ system and other effects:5 Ketamine (Ketalar) pharmacology: a summary of organ system and other effects:5
-
CNS action: Ketamine (Ketalar) induces a unique
anesthetic state referred to as dissociative anesthesia in which
the patient may appear "awake" or as is frequently
described "cataleptic".
-
Specific characteristics:
-
Significant analgesia and subanesthetic doses still
provide analgesia
-
Eyes remain open following administration
with cough, swallow, and corneal reflexes present.
-
Amnestic properties are present but less
than that observed with benzodiazepines, e.g. midazolam (Versed)
-
Anesthesia induction characteristics:
-
Increased limb muscle tone
-
Salivation, lacrimation, nystagmus,
pupillary dilatation
-
CNS metabolic effects: increased metabolism,
blood flow, and intracranial pressure
-
Increased electroencephalographic activity
-
 Emergence syndrome: There is a significant
likelihood (10%-30%) that the patient will experience unusual
psychological reactions to ketamine (Ketalar)
anesthesia. Emergence syndrome: There is a significant
likelihood (10%-30%) that the patient will experience unusual
psychological reactions to ketamine (Ketalar)
anesthesia.
-
Pulmonary effects are very limited.
-
 Cardiovascular effects: The stimulant
characteristics of ketamine (Ketalar) are manifest in
cardiovascular responses that seem opposite to that observance
most anesthetics. Cardiovascular effects: The stimulant
characteristics of ketamine (Ketalar) are manifest in
cardiovascular responses that seem opposite to that observance
most anesthetics.
-
For example, ketamine (Ketalar)
administration increases heart rate, cardiac output, and blood
pressure.
-
These effects may be relatively contraindicated in
patients sensitive to the expectable increase in myocardial oxygen
consumption.
-
Drugs can reduce these positive chronotropic
and hypertensive effects.
-
Etomidate (Amidate) Overview:
5,6
-
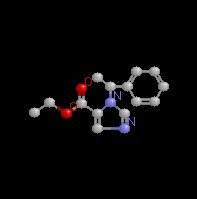
Etomidate (Amidate) which chemically is a carboxylated imidazole
derivative is an effective IV anesthetic agent which exhibits
favorable hemodynamic properties with minimal respiratory
depression.
-
This drug produces rapid unconsciousness (within about 30
seconds) following IV administration.
-
 Adverse effects, however, have resulted in reduced clinical
use. Adverse effects, however, have resulted in reduced clinical
use.
-
These adverse effects have included injection site pain
(which may be prevented by local anesthetic preinjection),
thrombophlebitis, myoclonus, nausea and vomiting, and inhibition
of steroid synthesis.
-
Nausea and vomiting may be especially
associated with etomidate (Amidate) compared to other induction
drugs and is made worse by concurrent use of opioids.
-
Etomidate (Amidate), a water insoluble drug which must be
dissolved in propylene glycol (35%; pH 6.9) has a chiral carbon,
resulting into enantiomers (stereoisomers) of which only one
enantiomer is active.
-
Etomidate
|
 |
-
Etomidate (Amidate)
pharmacokinetics:
5,6
-
Metabolism: Etomidate (Amidate) is metabolized by ester
hydrolysis (hepatic & tissue) as well as N-dealkylation.
-
Etomidate (Amidate) administration results in rapid onset,
follow by an initial redistribution phase which is also rapid
(initial redistribution halftime = 2.7 minutes).
-
Analysis of the
concentration-decay curve suggests that a three-compartment model
best fits the observed time dependent drop in plasma etomidate (Amidate)
concentration.
-
However, the initial rapid redistribution
time is most pertinent for explaining the observed rapid recovery
following IV administration.
-
Etomidate (Amidate) clearance ranges from 18-25 ml/kg/min.
-
Vd is large, consistent with a relatively lipophilic
compound which gains access to many compartments.
-
Rapid onset following IV administration is typical it has been
described as "one arm-brain circulation time".
-
Etomidate (Amidate) pharmacology
-
Summary of
etomidate effects organ system:
5,6
-
CNS:
-
Similar to observations concerning
thiopental (Pentothal) and other barbiturates, etomidate (Amidate)
while producing hypnosis does not produce analgesia.
-
Cerebral metabolism is reduced as well a
cerebral blood flow following etomidate (Amidate); these
effects result in a more favorable cerebral oxygen supply over
demand ratio.
-
Activation of the EEG following the etomidate (Amidate)has been observed and this property may be the basis
for epileptogenic activity.
-
Pulmonary:
-
Ventilation is depressed less
with etomidate (Amidate) compared to barbiturates, but apnea may
follow from rapid IV etomidate (Amidate) administration.
-
Importantly, given that etomidate (Amidate) may be administered
concommittantly with an opioid (or inhaled anesthetic),
respiratory depression can occur as a result of these
combinations.
-
Cardiovascular: An important distinction between etomidate (Amidate) and other induction agents is that etomidate (Amidate)
has very minimal cardiovascular effects.
-
Furthermore, during
induction blood flow to the heart and oxygen consumption are both
reduced which allows maintenance of the balance between oxygen
supply and requirement.
-
Since etomidate (Amidate) does not alter
sympathetic or baroreceptor reflex function, undesirable
hemodynamic effects may be induced by intubation.
-
Accordingly, an opioid (perhaps fentanyl (Sublimaze)), as
noted above, maybe given along with etomidate (Amidate).
-
Endocrine: Etomidate (Amidate) will cause
postoperative suppression of adrenocortical function.
-
This
effect occurs because etomidate inhibits 11-ß-hydroxylase and
17-α-hydroxylase enzymes which are important in cortisol
synthesis.
-
Short-term adrenocortical suppression as
might occur following single induction doses is not thought to
be clinically serious.
|