|
Pharmacokinetics: Opioids
-
Overview:
Opioids5,6:
-
Opioids are commonly used in IV
anesthesia. Contemporary agents include fentanyl (Sublimaze) and
fentanyl (Sublimaze) derivative such as alfentanil (Alfenta),
sufentanil (Sufenta), and remifentanil (Ultiva).
-
A primary opioid effect a
pharmacological interest is analgesia; however, there are a number
of important side effects consider in anesthesia.
-
For example, morphine can
cause hemodynamic effects including prominent vasodilation (venodilation)
which may be sufficient to require transfusion, hypotension or
even hypertension.
-
Fentanyl (Sublimaze),a
frequently used agent, exhibits respiratory depression, but
does not induce venodilation or histamine release
-
Fentanyl (Sublimaze)
derivatives, sufentanil (Sufenta) and alfentanil (Alfenta), are
characterized by more rapid recovery following IV infusion as
their plasma concentration decline more rapidly than that
observed with fentanyl (Sublimaze).
-
The shortest acting
agent, remifentanil (Ultiva), because of rapid hydrolysis, has
an exceedingly short half-life and may require continuous IV
infusion to maintain effect.
-
Mechanism of action:
-
Opioids work
by interacting with specific opiate receptors that have been
designated mu (μ), kappa (κ), and sigma (σ), in terms of major classification
types.
-
The mu
receptor type consists of the least two subtypes, μ1 which
probably mediates analgesia and μ2 which probably mediates
respiratory depression, bradycardia, and physical dependence.
-
The G protein second messenger
system is activated by opioid agonist binding to the
receptor.
-
Sites of opioid effects
include:
-
Medulla
-
Spinal cord
-
Spinal trigeminal nucleus, and
-
Periaquaductal grey area, an integration modulation
site from peripheral nerves to the central neuraxis.
-
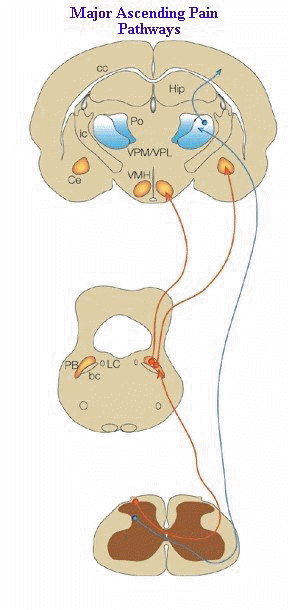
"There are two primary ascending nociceptive pathways.
-
These
are the spinoparabrachial pathway (red), which originates from the superficial
dorsal horn and feeds areas of the brain that are concerned with affect, and
the spinothalamic pathway (blue), which probably distributes nociceptive information
to areas of the cortex that are concerned with both discrimination and affect.
-
Many more less prominent pathways could be added2, 5, 6, 68-72.
-
(A,
adrenergic nucleus; bc, brachium conjunctivum; cc, corpus
callosum; Ce, central nucleus of the amygdala; Hip,
hippocampus; ic, internal capsule; LC, locus coeruleus; PB,
parabrachial area; Po, posterior group of thalamic nuclei; Py,
pyramidal tract; RVM, rostroventral medulla; V, ventricle; VMH,
ventral medial nucleus of the hypothalamus; VPL, ventral
posteriolateral nucleus of the thalamus; VPM; ventral
posteriomedial nucleus of the thalamus.)"
-
Figure adapted from: Nature Reviews Neuroscience 2;
83-91
(2001): THE MOLECULAR DYNAMICS OF PAIN CONTROL Nature © Macmillan Publishers Ltd 2001 Registered No. 785998 England
|
|
-
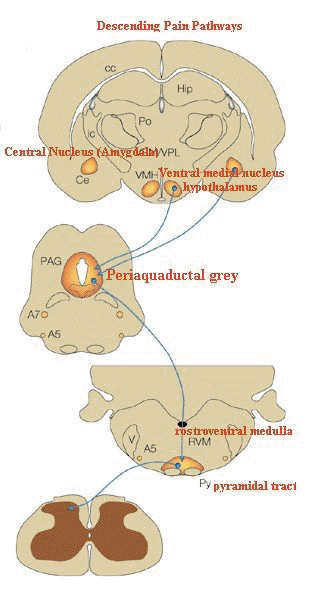
"The descending pathway highlighted
originates from the amygdala and hypothalamus and terminates
in the periaqueductal grey (PAG).
-
Neurons project from here to
the lower brainstem and control many of the antinociceptive
and autonomic responses that follow noxious stimulation.
-
(A,
adrenergic nucleus; bc, brachium conjunctivum; cc, corpus
callosum; Ce, central nucleus of the amygdala; Hip,
hippocampus; ic, internal capsule; LC, locus coeruleus; PB,
parabrachial area; Po, posterior group of thalamic nuclei; Py,
pyramidal tract; RVM, rostroventral medulla; V, ventricle; VMH,
ventral medial nucleus of the hypothalamus; VPL, ventral
posteriolateral nucleus of the thalamus; VPM; ventral
posteriomedial nucleus of the thalamus.)"
-
Figure adapted from: Nature Reviews Neuroscience 2;
83-91
(2001): THE MOLECULAR DYNAMICS OF PAIN CONTROL Nature © Macmillan Publishers Ltd 2001 Registered No. 785998 England
|
|
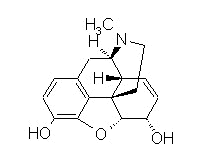
Morphine
|
|
|
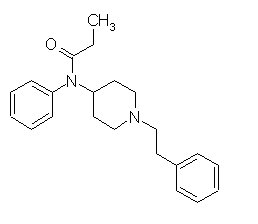
Fentanyl (Sublimaze)
|
 |
|
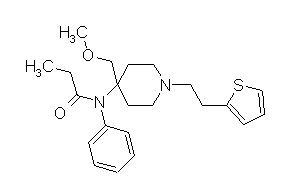
Sufentanil (Sufenta)
|
|

|
-
Since opioid biological actions require the
molecule to interact with specific receptors and in the case of
anesthesia these receptors of primary interest are localized in
the spinal cord and brain, the ionization state of the drug
is a critical importance. As noted earlier, it is the
un-ionized form that more readily moves from the aqueous phase
into of the lipid or as we see below "non-polar"
component of the membrane. These non-polar tails form
the transport barrier for polar or formally charged
molecules.
|
Membrane Bilayer
Structure
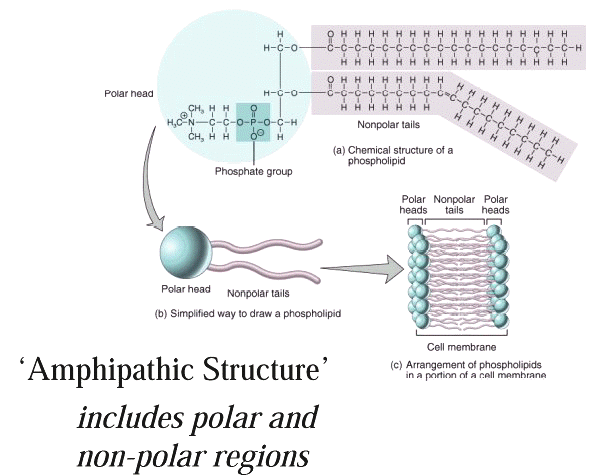
Representation of the lipophilic,
hydrophobic core characteristic of biomembrane
structure.
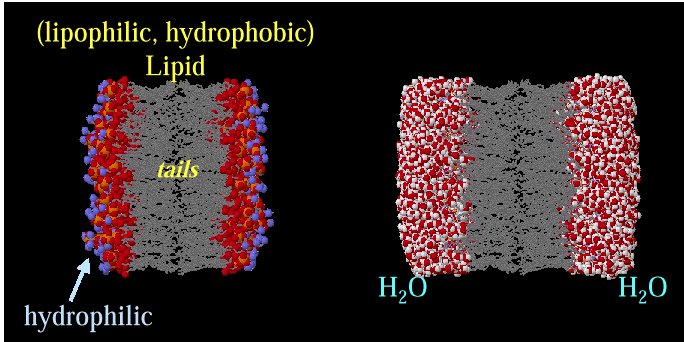
Above images courtesy of Professor Steve Wright and the University
of Arizona (c) 2001, used with permission.
|
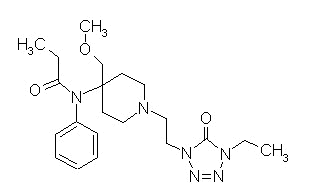
Alfentanil
|
|
|
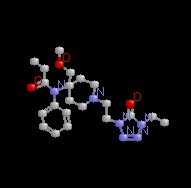
Remifentanil (Ultiva)
|
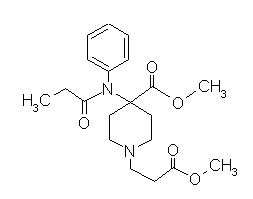
Remifentanil |
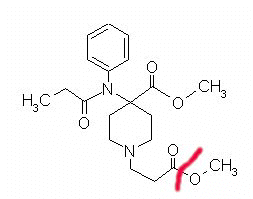
Remifentanil showing hydrolysis site
|
Remifentanil (Ultiva)
|

|
|
*Opioid Organ system Effects:
see section on Opioid Pharmacology in the Central Nervous
System Unit
|