|
Principal Organs for Biotransformation:
Correia, M.A., Drug Biotransformation. in Basic and Clinical Pharmacology,
(Katzung, B. G., ed) Appleton-Lange, 1998, pp 50-61.
Benet, Leslie Z, Kroetz, Deanna
L. and Sheiner, Lewis B The Dynamics of Drug Absorption,
Distribution and Elimination. In, Goodman and Gillman's
The Pharmacologial Basis of Therapeutics,(Hardman, J.G, Limbird, L.E,
Molinoff, P.B., Ruddon, R.W, and Gilman, A.G.,eds) TheMcGraw-Hill Companies, Inc.,1996, pp. 3-27
Mixed function oxidase
System (cytochrome 450 System)--Phase I
Reactions
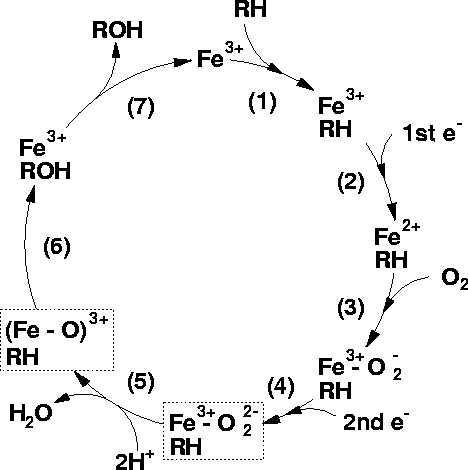
Cytochrome p450 cycle (diagram by Matthew Segall,
1997)
-
"The binding of a substrate to a P450 causes a lowering of the redox potential by approximately 100mV, which makes the transfer of an electron favourable from its redox partner, NADH or
NADPH.
-
The first reduction -The next stage in the cycle is the reduction of the
Fe3+ ion by an electron transfered from NAD(P)H via an electron transfer chain.
-
Oxygen binding An O2
molecule binds rapidly to the ion Fe2+ forming
Fe2+-O2
-
Second reduction A second reduction is required by the stoichiometry of the reaction. This has been determined to be the rate-determining step of the reaction
-
O2 cleavage: The O2
reacts with two protons from the surrounding solvent, breaking the O-O bond, forming water and leaving an
Fe-O3+ complex.
-
Product formation The Fe-ligated O atom is transferred to the substrate forming an hydroxylated form of the substrate.
-
Product release The product is released from the active site of the enzyme which returns to its initial state."--Matthew
Segall, 1997
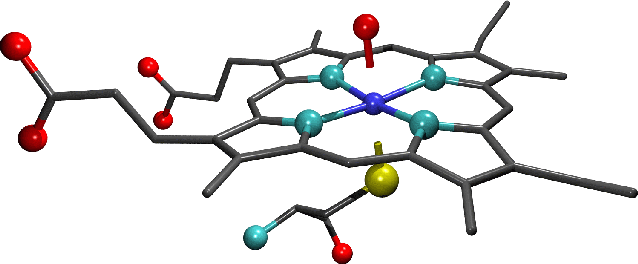
-
"The active site of substrate-free cytochrome
p450:Note the water molecule (which can be seen as a single oxygen atom) that forms the sixth axial ligand of the
haem iron. Oxygen atoms are shown in red, nitrogen in light blue, sulphur in yellow and
iron in dark blue. Carbon atoms are shown in grey as bonds only and
hydrogens have been omitted from this figure for clarity."
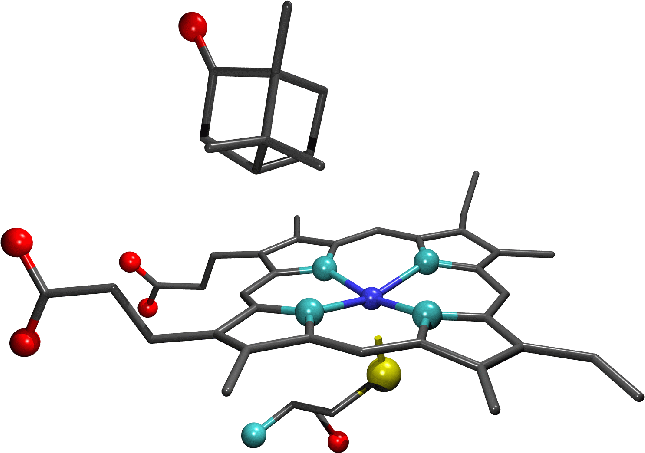
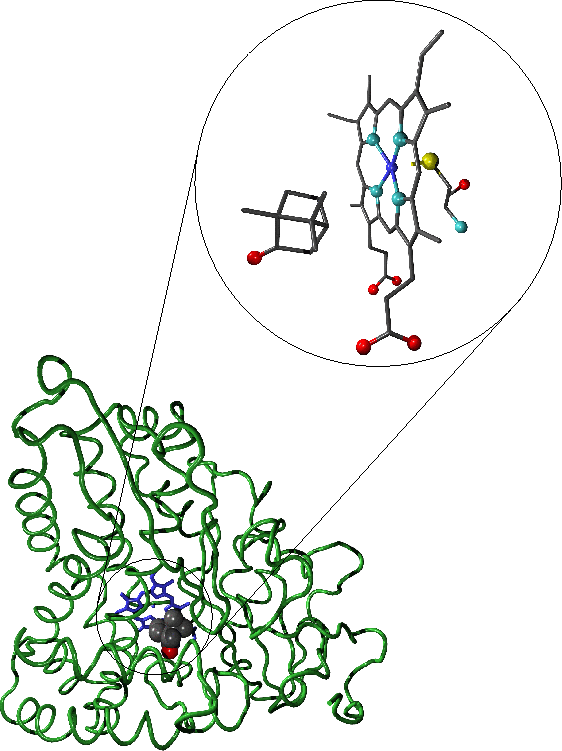
-
"A representation of with bound camphor. The enlarged active site region shows the camphor substrate, haem moiety and cysteine residue which
forms the distal haem ligand. In the representation of the full enzyme the protein backbone is shown in green, the haem moiety in blue and the substrate is coloured
according to atomic species. Oxygen atoms are shown in red, carbon in grey, nitrogen in light blue, sulphur in yellow and iron in dark blue."-diagrams
and text by Matthew Segall, 1997
-
Cytochrome P450 Enzyme Induction:
-
Cytochrome P450 enzyme inhibition:
-
Certain drugs, by binding
to the cytochrome component, act to
competitively inhibit metabolism.
Examples:
-
Catalytic inactivation of cytochrome P450.
-
Macrolide antibiotics (troleandomycin,
erythromycin estolate (Ilosone)),
metabolized by a cytochrome P450:
-
Chloramphenicol (Chloromycetin):
metabolized by cytochrome P450 to
an alkylating metabolite that
inactivates cytochrome P450
-
Other inactivators:
Mechanism of Action: -- targeting
the heme moiety:
-
steroids:
-
others:
-
propylthiouracil
-
ethchlorvynol (Placidyl)
Phase II
Metabolism
Some Phase II Reactions
|
Type of
Conjugation
|
Endogenous
Reactant
|
Transferase
(Location)
|
Types of
Substrates
|
Examples
|
|
Glucuronidation
|
UDP glucuronic
acid
|
UDP
glucuronosyl transferase (microsomal)
|
phenols,
alcohols, carboxylic acids, hydroxylamines, sulfonamides
|
morphine,
acetaminophen, diazepam, digitoxin, digoxin, meprobamate
|
|
Acetylation
|
Acetyl-CoA
|
N-Acetyl
transferase (cytosol)
|
Amines
|
sulfonamides,
isoniazid, clonazepam, dapsone, mescaline
|
|
Glutathione
conjugation
|
glutathione
|
GSH-S-transferase
(cytosolic, microsomes)
|
epoxides, nitro
groups, hydroxylamines
|
ethycrinic
acid, bromobenzene
|
|
Sulfate
conjugation
|
Phosphoadenosyl
phosphosulfate
|
Sulfotransferase
(cytosol)
|
phenols,
alcohols, aromatic amines
|
estrone,
3-hydroxy coumarin, acetaminophen, methyldopa
|
|
Methylation
|
S-Adenosyl-methionine
|
transmethylases
(cytosol)
|
catecholamines,
phenols, amines, histamine
|
dopamine,
epinephrine, histamine, thiouracil, pyridine
|
|
Adapted from Table 4-3, Correia,
M.A., Drug Biotransformation. in Basic and Clinical
Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998,
p 57. |
Nonspecific esterases in liver, plasma,
gastrointestinal tract hydrolyzed drugs containing
ester linkages, e.g.:
|
succinylcholine (Anectine) |
atracurium
(Tracrium) |
mivacurium
(Mivacron) |
esmolol
(Brevibloc) |
Ester-type
local aesthetics |
|
Correia, M.A., Drug
Biotransformation. in Basic and Clinical Pharmacology,
(Katzung, B. G., ed) Appleton-Lange, 1998, pp 50-61. |
|
Benet, Leslie Z, Kroetz, Deanna
L. and Sheiner, Lewis B The Dynamics of Drug Absorption,
Distribution and Elimination. In, Goodman and Gillman's
The Pharmacologial Basis of Therapeutics,(Hardman, J.G,
Limbird, L.E, Molinoff, P.B., Ruddon, R.W, and Gilman,
A.G.,eds) TheMcGraw-Hill Companies, Inc.,1996, pp. 3-27 |
|
Stoelting, R.K.,
"Pharmacokinetics and Pharmacodynamics of Injected
and Inhaled Drugs", in Pharmacology and Physiology
in Anesthetic Practice, Lippincott-Raven Publishers,
1999, 1-17. |
|