Nursing Pharmacology: Antiviral Drugs
Antiviral Drugs
Anti-viral drugs with activity against HIV (Human Immunodeficiency Virus)
HIV-1 Pathophysiology/Pathogenesis
![]() The
immunodeficiency at the heart of HIV-1 viral disease occurs as a result
of targeting by the virus of a subset of T-cell lymphocytes described as
helper T cells.2
The
immunodeficiency at the heart of HIV-1 viral disease occurs as a result
of targeting by the virus of a subset of T-cell lymphocytes described as
helper T cells.2
These helper T cells are described by CD4+ molecules present on its surface and it is these molecules which serve as the principal HIV-1 cellular receptor.2
As described earlier, a coreceptor must also be present along with CD4+ in support of binding, fusion and HIV-1 entry into target cells.
Two major co-receptors, CCR5 and CXCR4, are utilized by HIV.
Furthermore these co-receptors act as primary receptors for some chemoattractant cytokines (chemokines).
These co-receptors are classified as belonging to the family of seven-transmembrane domain G protein-coupled receptors.2
Destructive effects of HIV-1 are mediated both by direct interaction as well as by indirect effects, including, immunological clearance of infected cells and activation-induced cell death, such as pyroptosis.
Associated with declining CD4+ T cells is the development of a category of diseases manifested as opportunistic infections and neoplasms.2
These are "AIDS-defining" illnesses.
However, some manifestation of AIDS, including Kaposi's sarcoma and some neurological effects may be incompletely explained by HIV-1-mediated immunodeficiency, given that these complications appear to occur prior to severe immunological impairment.
The timeframe depicting the relationship between declining CD4+ T cell count, clinical latency, and opportunistic disease is described below.2
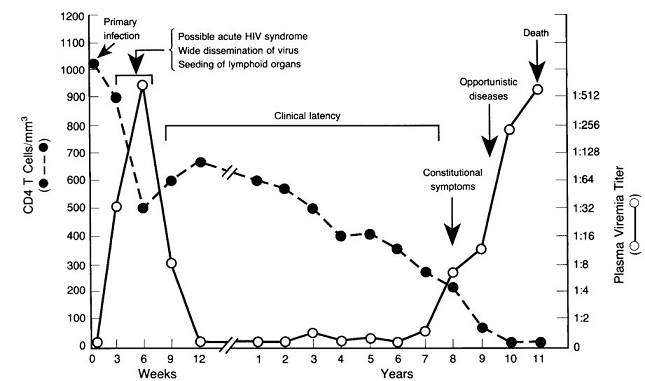 |
|
Initial Events in HIV-1 Infection:
Entry of HIV-1 through the intact or degraded mucosal barrier or by dendritic cell transport is followed by viral association with target cells.2
 |
|
|
|
![]() The major
HIV target is typically CD4+ T cells.2
The major
HIV target is typically CD4+ T cells.2
|
|
|
"Partially" resting CD4+ T cells as well as activated CD4+ T cells amplify early infection.
Although resting CD4+ T cells are greater in number compared to activated CD4+ T cells, the latter subset is able to produce more virus.
During the initial days to weeks following infection, HIV-1 virus is found initially in draining lymph nodes before proceeding to other lymphoid compartments, there gaining access to elevated concentration of CD4+ T cell targets.
Access to this enriched CD4+ T cell environment accounts for an early burst of high-level viremia.2
Gut-associated lymphoid tissue (GALT) is an important HIV-1 viral target and development of HIV-1 infection in GALT infection depletes memory-cell enriched CD4+ T cell subpopulations.
Depletion of these cells occurs both by direct virus infection and virus-induced apoptosis.2
![]() At
this point, with the infection established, continued HIV
replication and dissemination appear irreversible.2
At
this point, with the infection established, continued HIV
replication and dissemination appear irreversible.2
Some characteristics of initial target cell infection may be dependent on the route of infection.
For example, virus entering the bloodstream directly (e.g. via blood/blood products) may be cleared by normal physiological processes to the spleen and other lymphoid organs.
![]() At
these sites primary focal infections begin and wider
spread to other lymphoid tissues follow.2
At
these sites primary focal infections begin and wider
spread to other lymphoid tissues follow.2
HIV-1 Resistance to Antibody Neutralization:
HIV sexual transmission has been described as a single infectious event.2
Features of the HIV envelope glycoprotein appear to influence significantly HIV transmission (for subtype A and C viruses, at least).2
![]() The
viral isolate most active in transmission has been designated "founder
viruses," and are less common in the viral population of the
transmitting partner and also appear less divergent with respect to
certain specific sequences.
The
viral isolate most active in transmission has been designated "founder
viruses," and are less common in the viral population of the
transmitting partner and also appear less divergent with respect to
certain specific sequences.
![]() The
HIV-1 viral spike represents the sole viral target for neutralizing
antibodies and has evolved to counteract antibody-mediated
neutralization.11
The
HIV-1 viral spike represents the sole viral target for neutralizing
antibodies and has evolved to counteract antibody-mediated
neutralization.11
![]() As
replication proceeds in the newly infected individual, the founder virus
diverges, variation in glycosylation sites results in increasing
resistance to antibody neutralization.2,13
As
replication proceeds in the newly infected individual, the founder virus
diverges, variation in glycosylation sites results in increasing
resistance to antibody neutralization.2,13
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |