Medical Pharmacology Chapter 35 Antibacterial Drugs
First Generation Cephalosporins
Cephalexin: Audio Overview
 |
 |
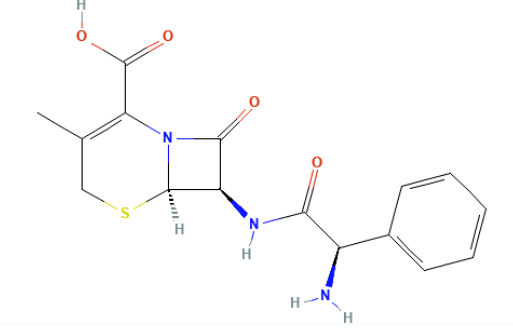
Cephalexin is a first-generation β-lactam cephalosporin antibiotic that serves as fundamental agent for treatment of community-acquired infections due to susceptible Gram-positive organisms and select Gram-negative bacteria.
The clinical significance of cephalexin lies in its oral bioavailability, favorable safety profile, and use across various patient populations, including children, adults, and pregnant individuals.2,3,4,5,6
Cephalexin Mechanism of Action
![]() Cephalexin
exerts a bactericidal effect
by inhibiting bacterial cell wall synthesis.
Cephalexin
exerts a bactericidal effect
by inhibiting bacterial cell wall synthesis.
Bactericidal effects occur as result of acylation of penicillin-binding proteins (PBPs), crucial enzymes in the final transpeptidation step of peptidoglycan synthesis.
The transpeptidation step is essential for proper structural framework which provides rigidity and shape to bacterial cell walls.
![]() Inhibition
of this step prevents the cross-linking of peptidoglycan
strands, leading to mechanical instability, cell wall
degradation, and ultimately osmotic
lysis
of the bacterial cell.2,3,7
Inhibition
of this step prevents the cross-linking of peptidoglycan
strands, leading to mechanical instability, cell wall
degradation, and ultimately osmotic
lysis
of the bacterial cell.2,3,7
Bacterial resistance can develop:
1. Through production of β-lactamase enzymes (which hydrolyze the β-lactam ring)
2. Alterations in PBPs reducing drug affinity, or
3. Via efflux mechanisms that expel the antibiotic from bacterial cells.2
Absorption, Distribution
Cephalexin is acid-stable and thus well absorbed orally, with bioavailability approaching 90–100%.
![]() Well
absorbed after oral administration, cephalexin achieves peak
plasma concentration within 1 hour
whether or not food is present.
Well
absorbed after oral administration, cephalexin achieves peak
plasma concentration within 1 hour
whether or not food is present.
The drug is 10–15% protein bound, distributing widely to tissues and fluids such as bone, skin, respiratory secretions, and urine.
![]() Penetration
into cerebrospinal fluid is poor, limiting its role in
treating meningitis.2,3,8
Penetration
into cerebrospinal fluid is poor, limiting its role in
treating meningitis.2,3,8
Metabolism
![]() Cephalexin
undergoes minimal
hepatic metabolism,
and more than 90%
is excreted unchanged in the urine via
glomerular filtration and tubular secretion.
Cephalexin
undergoes minimal
hepatic metabolism,
and more than 90%
is excreted unchanged in the urine via
glomerular filtration and tubular secretion.
As elimination half-life ranges from 0.5 to 2 hours, dosing is every 6–8 hours for optimal therapeutic levels.
![]() Because
excretion is primarily renal, dose adjustment is essential
in patients with renal impairment to prevent accumulation
and toxicity.
Because
excretion is primarily renal, dose adjustment is essential
in patients with renal impairment to prevent accumulation
and toxicity.
![]() Co-administration with probenecid can
prolong plasma levels by inhibiting renal excretion.2,6,8,9
Co-administration with probenecid can
prolong plasma levels by inhibiting renal excretion.2,6,8,9
Cephalexin Therapeutic Uses4,5
Cephalexin is active against:
Most Gram-positive cocci, including:
Streptococcus pyogenes and
Methicillin-susceptible Staphylococcus aureus (MSSA)
And select Gram-negative organisms such as:
Proteus mirabilis
Escherichia coli, and
Klebsiella pneumoniae.
![]() Clinical
Uses5,10
Clinical
Uses5,10
Skin and soft tissue infections such as cellulitis, wound infections, and abscesses.
Pharyngitis and tonsillitis due to group A streptococci.
Urinary tract infections (uncomplicated).
Otitis media and sinusitis.
Bone and joint infections (less commonly used in the current clinical setting).
Bacterial endocarditis prophylaxis in some circumstances for penicillin-allergic individuals.
![]() Cephalexin
remains a mainstay of oral therapy for mild to
moderate community-acquired infections when
intravenous agents like cefazolin are
unnecessary.11
Cephalexin
remains a mainstay of oral therapy for mild to
moderate community-acquired infections when
intravenous agents like cefazolin are
unnecessary.11
Cephalexin is usually well tolerated, with adverse reactions reflecting its β-lactam class profile.
Gastrointestinal reactions such as nausea, vomiting, diarrhea, and abdominal pain represent the most frequently encountered adverse effects.
Hypersensitivity reactions range from mild rashes and urticaria to severe anaphylaxis in penicillin-allergic individuals, though cross-reactivity risk is ≤10%.
![]() Clostridioides difficile-associated
diarrhea represents a potentially serious
complication of broad-spectrum antibiotic use.4,12
Clostridioides difficile-associated
diarrhea represents a potentially serious
complication of broad-spectrum antibiotic use.4,12
Other possible reactions include eosinophilia, transient hepatic enzyme elevation, and interstitial nephritis, though these are rare.
Cephalexin is considered safe during pregnancy and lactation.4,12
Clinical Use Summary
Cephalexin offers a reliable oral option bridging outpatient and inpatient antibiotic treatment.
Its narrow spectrum against skin flora makes it ideal for de-escalation after empiric intravenous therapy using broader-spectrum drugs.
Cephalexin's role remains critical in community settings where susceptibility to first-generation cephalosporins persists—notably against MSSA and streptococcal infections.5,6
Cephalexin lacks activity against methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus, and most Pseudomonas species, which confines its role primarily to infections lacking these resistant pathogens.
Cephalexin is noted for optimal narrow-spectrum therapy and represents an older but still clinically important antibiotic.4,5,11
November 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |