Overview
return to
main menu
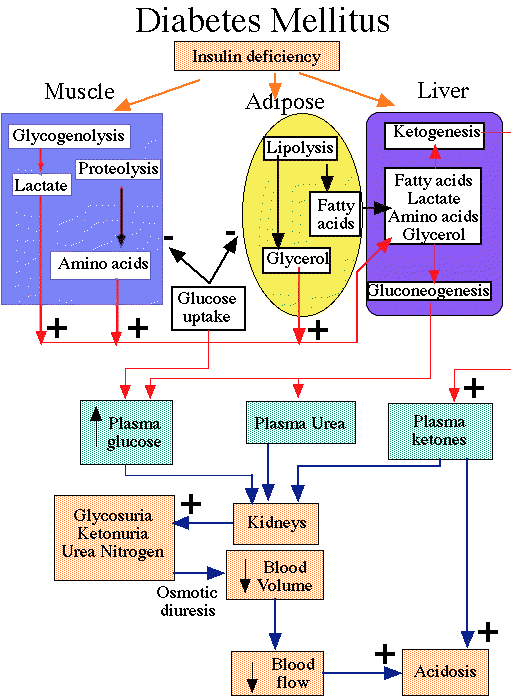
- "Effects of Insulin deficiency"
- courtesy of Robert H. Parsons, Ph.D.,
Rensselaer Polytechnic Institute, used with permission
return
to main menu
Properties
of IDDM* and NIDDM**
| Characteristic |
IDDM |
NIDDM |
Genetic
locus
|
Chromosome
6
|
unknown
|
Typical
age of onset
|
Usually
< 40 years of age
|
> 40
years of age
|
Plasma
insulin
|
Low to
absent
|
Normal
to high
|
Plasma
glucagon
|
High,
suppressible
|
High,
resistant
|
Acute
complication
|
Ketoacidosis
|
Hyperosmolar
coma
|
Insulin
therapy
|
Responsive
|
Responsive
to resistant
|
Response
to sulfonylurea drugs
|
Unresponsive
|
Responsive
|
* Insulin-dependent
diabetes mellitus
**Non-insulin dependent diabetes
mellitus
return to
main menu
Type II diabetes:
- a group of milder forms of
diabetes
- occurring mainly in adults
- endogenous insulin:
sufficient to prevent ketoacidosis --
- abnormal insulin
secretion
- resistance to insulin
action at the tissue.
- Obesity: common risk factor
- NIDDM patients: deficiency in
pancreatic B cell response to glucose
- impaired response
worsened by hyperglycemia
Pathophysiological Phases:
-
plasma
glucose: normal -- elevated
insulin levels
-
elevated
insulin levels -- postprandial
hypoglycemia
-
Insulin or
systems to change -- reduced
insulin secretion causes fasting
hyperglycemia him (diabetes)
Clinical Presentations:
-
NIDDM: appears in middle
age or later; mainly in
overweight patients
-
gradual onset of
symptoms
-
Presenting
symptoms:
-
extreme hyperglycemia
-
hyperosmolality
-
volume depletion
-
CNS symptoms (ranging
from clouded sensorium to
coma)
-
seizure
activity (Jacksonian)
-
transient
hemiplegia
-
Infections -- pneumonia
and gram-negative sepsis
(common, associated with
very negative prognosis)
-
patients with
NIDDM: do not develop
ketoacidosis
-
patients with NIDDM:
may develop hyperosmolar,
nonketotic coma
-
Hyperosmolar coma
can also be caused by:
-
peritoneal/hemodialysis
-
tube
feeding of high-protein
formulas
-
high-carbohydrate
in fusion loads
-
osmotic
agents (mannitol and
urea)
-
Prognosis:
-
Treatment of
hyperosmolar coma states:
-
large
amounts of intravenous
fluids (average fluid
deficit: 10-11 liters.
-
Insulin:
more rapid control
hyperglycemia
-
Potassium
salts (counteract
intracellular shifted
plasma K+)
-
Sodium
bicarbonate (if lactic
acidosis present)
-
Antibiotics
return
to main menu
Insulin
Overview:
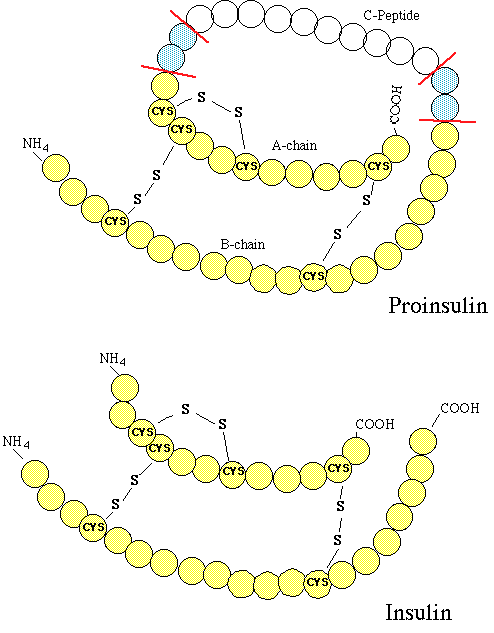
 small
protein,composed of two chains and( A & B) small
protein,composed of two chains and( A & B)- Pancreatic B cells produced proinsulin, the
insulin precursor, which consists of a single-chain protein
- Proinsulin, following Golgi apparatus processing,
is packaged into granules -- that hydrolyzes insulin and C-peptide
- Pancreatic B cell granules store insulin in
crystals (2 atoms of zinc six molecules of insulin)
- 28 units of insulin per milligram
return to main menu
-
Secretion:
-
normally low basal rate from pancreatic B
cells
-
higher stimulated rate in response to:
-
glucose
-
other sugars (e.g. mannose)
-
certain amino acids (e.g. leucine,
arginine)
-
vagal nerve activity
-
Proposed
secretion mechanism:
-
hyperglycemia
-
increased intracellular ATP concentration
-
higher intracellular ATP closes
ATP-dependent potassium channels
-
decreased outward potassium current
causes pancreatic B cell depolarization and opens
voltage-gated calcium channels
-
increased intracellular calcium promotes
insulin secretion
-
intracellular second messengers modulate
release:
-
cyclic AMP
-
inositol triphosphate
-
diacylglycerol
-
Insulin
Degradation:
return to main menu
-
Insulin
Receptor:
-
Insulin binds to target receptors (high
affinity, high specificity) and liver, muscle, and fat tissue
-
Insulin
receptor: -- composition
-
two heterodimers;
-
each containing an alpha subunit (extracellular:
recognition site) and
-
a beta subunit which spans the
membrane and contains a tyrosine kinase
-
Receptor
Activation:
-
insulin binds to alpha subunit
-
beta subunit increases tyrosine
kinase activity, resulting in auto- phosphorylation
-
Phosphorylated beta subunit
promotes aggregation of heterodimers and stabilizes the
receptor tyrosine kinase activated state
-
docking protein (insulin receptor
substrate-1, IRS-1) is then phosphorylated
-
phosphorylated IRS-1 activates
other kinases, promoting further phosphorylation reactions
-
Insulin's second messenger's:
these phosphorylation products
-
Insulin-receptor complex is then
internalized
-
Alteration
in Insulin receptor affinity:
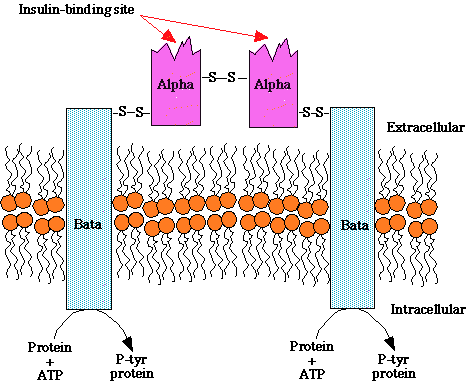
- "Structure of
the insulin receptor, a heterotetramer consisting of two
extracellular insulin-binding subunits linked by a disulfide bonds
to tow transmembrane beta subunits. The beta subunits contain an
intrinsic tyrosine kinase activity that is activated upon insulin
binding to the alpha subunit. "
- courtesy of Robert H. Parsons, Ph.D.,
Rensselaer Polytechnic Institute, used with permission
return to main menu
return to main menu
Insulin
preparations: Overview
-
Four principal
types:
-
ultra-short-acting-- (very rapid onset; short
duration)
-
short-acting --(rapid onset of action)
-
intermediate-acting
-
long acting -- (slow onset of action)
-
Insulin
modifications:
-
Ultra-short-acting
insulin:
-
Short-acting
insulin:
-
Intermediate-acting
and long acting insulins:
-
NPH
(neutral protamine Hagedorn or isophane) insulin:
-
intermediate-acting; onset of action delayed
by combining correct amounts of protamine insulin such that
neither is uncomplexed.-Onset and duration of action: NPH
insulin -- similar to lente insulin:
-
NPH insulin usually mixed with regular
insulin: for twice daily administration
-
Insulin Mixtures:
-
Insulin Species:
-
Beef and
pork insulins:
-
Most commercial insulin contained: beef
insulin -- primary component
-
Beef hormone: slightly more antigenic
compared to pork insulin
-
common ratio: 70% beef/30% pork insulin
-
Recombinant DNA techniques have allowed
mass production of human insulin
-
Human insulins:
-
Readily available: in regular, NPH, lente,
or ultralente form.been
-
Premixed formulation of 70% NPH and 30%
regular human insulin is available
-
Human insulin: Advantages
-
as effective as animal insulins
-
much less immunogenic than beef-pork
insulin; slightly less immunogenic import insulin
-
Human insulin: more rapidly absorbed;
slightly shorter duration of action
-
Human Proinsulin:
-
available through recombinant DNA synthesis
-
Proinsulin biological activity about 8-12%
that of human insulin
-
Four-six times longer circulating half-life
compared to human insulin.
-
Fatal cardiovascular reactions in a group of
patients receiving human proinsulin have suspended clinical
trials
-
Delivery systems:
-
portable pen injectors: replaced syringes
-
closed loop systems: blood glucose control a
insulin infusion
-
open-loop systems (insulin pumps)
-
nasal insulin delivery: poor absorption
return to main menu
-
Insulin
Treatment:overview
-
Glycemic
control in diabetes mellitus
-
Complications
of insulin treatment:
-
Insulin
Therapy: immunopathology
-
Insulin allergy: immediate type
hypersensitivity, rare
-
urticaria follows history release from
tissue mast cells (sensitized by anti-insulin IgE
antibodies) the subcutaneous nodule appears that injection
site {IgE-mediated complement-binding Arthus reaction}
-
antihistamines, corticosteroids, or
desensitization may be required
-
less common with new, highly purified
insulins
-
Immune insulin resistance:
-
due to high titer circulating IgG
anti-insulin antibodies
-
Very high IgG anti-insulin antibodies
require high insulin levels to compensate
-
with highly purified insulin, now
available, this reaction is very rare
-
Injection site lipodystrophy:
-
atrophy of subcutaneous fat
-
rare complication due to availability of
more highly concentrated insulin preparations of neutral
pH
-
Hypertrophy of subcutaneous fatty tissue
is a problem if insulin is injected repeatedly at the same
site -- liposuction can correct
return to main menu
Table:
Sulfonylureas
|
Tolbutamide (Orinase)
|
tolazamide (Tolinase)
|
acetohexamide
|
|
chlorpropamide (Diabinese)
|
glyburide (Micronase, DiaBeta)
|
glipizide (Glucotrol)
|
-
Tolbutamide: (Orinase)
-
well absorbed; shorter duration of action
-
safest sulfonylurea for use in the elderly
-
rare acute toxic reactions
-
some drug drug interactions (dicumarol,
phenylbutazone, or some sulfonamides)
-
Tolazamide (Tolinase)
-
comparable to chlorpropamide in potency;
shorter duration of action
-
slow absorption; delayed effect on blood
glucose
-
metabolized to biologically active
compounds
-
Acetohexamide:
return to main menu
Biguanides
Alpha-glucosidase
Inhibitor
-
Acarbose (Precose)
-
oligosaccharide analog -- strongly binds to
intestinal disaccharidases (e.g. alpha-glucosidase)
-
results in delayed carbohydrate absorption:
-
Adverse
Effects:
-
Drug-drug interaction: acarbose
interferes with metformin absorption
-
Miglitol (Glyset)
return to main menu
Thiazolidinedione
Derivatives
return to main menu
Glucagon
return to main menu
|