|
|
|
|
-
-
There are several mechanisms by which drugs move through the body and arrive at the intended site of action.
-
 These mechanisms
include:12
These mechanisms
include:12 -
Aqueous Diffusion
-
Lipid Diffusion
-
Membrane Transporters (by active transport or by facilitated diffusion)
-
Endocytosis and Exocytosis12
-
These mechanisms involve translocation of drugs across membranes.8
-
-
 Most of the time
the drug directly crosses membranes in accord with certain drug and
membrane intrinsic properties.
Most of the time
the drug directly crosses membranes in accord with certain drug and
membrane intrinsic properties. -
Since biological membranes have a lipid core, some drugs which are not lipid-soluble have difficulty crossing membranes.8
-
These drugs may have a nonuniform electrical charge distribution, with one part of the molecule relatively more positive or negative compared to another part of molecule, i.e. a "polar molecule."
-
An example of this principle is illustrated in a water molecule.8
-
Water Molecule Dipole Moment16,8 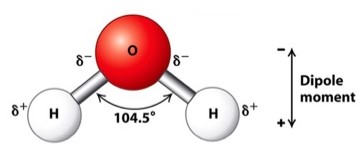
-
Image Attribution:
-
Note that the oxygen (O) is relatively negatively charged and so noted by the use of the designation δ- .
-
By contrast, the hydrogen atoms (H) are relatively positively charged and designated by the term δ+ .
-
These charges are not formal charges as for example in sodium ion (Na+) which is explicitly electron deficient.
-
-
-
This same principle of relative charge distribution can also be seen in complex molecules (drugs)
-
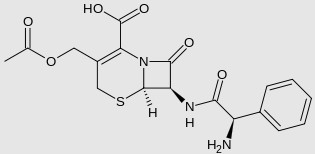
Examples of more complex, polar drugs include: gentamicin, cephaloglycin (a.k.a. cefaloglycin; a seldom used first-generation cephalosporin antibiotic), and floxuridine (5-fluorodeoxyuridine; an anticancer drug in the antimetabolite class, often used in the management of colorectal cancer).
-
-
Polar Drugs17 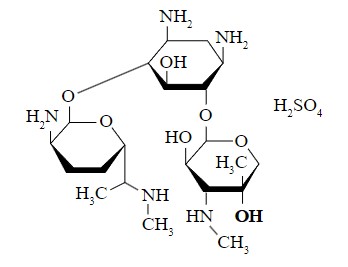
Gentamicin
-
Attribution:
-
cLogP = -4.1
-
cLogP is a "partition coefficient", representing the drug concentration ratio in two different solvents.
-
The "c" refers to "calculated" whereas log refers to logarithm and P refers to "partition".
-
-
LogP is > 0 for lipophilic drugs and < 0 for hydrophilic (polar) drugs.
Cephaloglycin
-
Attribution: Fvasconcellos, Public domain, via Wikimedia Commons
-
cLogP = -3.0
-
-
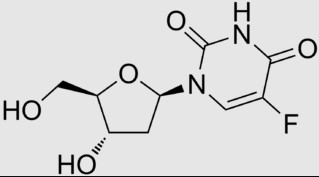
Floxuridine
-
Attribution: Edgar181, Public domain, via Wikimedia Commons
-
cLogP = -1.2
-
-
Gentamicin and the origin of intramolecuar polarity.8
-
The OH (hydroxyl) groups tend to attract electrons, making this part of the molecule more negative compared to other parts of the molecule.8
-
Gentamicin, a polar antibiotic drug
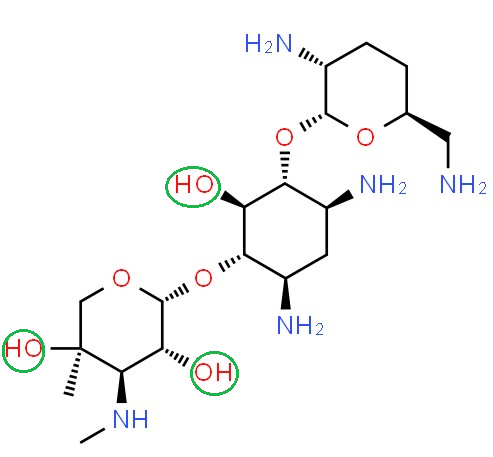
-
Image Attribution:
-
-
-
-
 Characterization of a drug as relatively nonpolar or polar may be based
upon a drug's partition coefficient, the ratio of the drug concentration
between two different solvents.
Characterization of a drug as relatively nonpolar or polar may be based
upon a drug's partition coefficient, the ratio of the drug concentration
between two different solvents.
-
Usually one of the solvents is water and the other is a nonpolar solvent, typically octanol (n-octanol).
-
Consider the separatory funnel illustrated below in which an oil layer (clear) is separated from the water layer (green).
-
Separatory Funnel  z
z-
Attribution:
-
PRHaney, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
-
https://commons.wikimedia.org/wiki/File:Separatory_funnel_with_oil_and_colored_water.jpg
-
-
-
-
 The
partition coefficient, P, describes then the difference in
solubility of the drug in non-mixing (immiscible) solvents:
water vs. n-octanol (ontan-1-ol).18
The
partition coefficient, P, describes then the difference in
solubility of the drug in non-mixing (immiscible) solvents:
water vs. n-octanol (ontan-1-ol).18
-
The partition coefficient describes lipophilicity for neutral drugs or for the case in which the drug exists in a single form.
-
-
Partition Coefficient, P = [drug]n-octanol / [drug]water
-
LogP = log 10 (Partition Coefficient)
-
For example, if LogP = 1 then the ratio of drug concentration in the organic phase compared to the aqueous (water) phase = 10.
-
-
-
For those drugs that can exist as different species (different ionized states) depending on the pH, the distribution of the species between the n-octanol and water phases is described by a Distribution coefficient which takes into account the concentration of individual species in the two phases.18
-
LogP vs. LogD18 -
LogD is appropriately used in describing lipophilicity of ionizable drugs, given that the pH dependency of the molecule in water is taken into account.
-
LogP is the correct way of describing lipophilicity for neutral compounds.
-
-
-
-
-
-
Reviewed June 2025
|
|
References
Geroge Jr AL Neilson EG Chapter 309 Cell Biology and Physiology of the Kidney in Harrison's Principles of Internal Medicine (Loscalzo J Kasper DL Longo DL Fauci AS Hauser SLs Jameson JL, eds) 21e 2022. Burchum JR Rosenthal LD Charles C Chapter 4 Pharmacokinetics Lehne's Pharmacology for Nursing Care 11e Elsevier 2022. Singer SJ Nicolson GL The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972 Feb 18; 175(4023): 720-731. Watson H Biological membranes Essays Biochem (2015) 59, 43-70. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626904/pdf/bse0590043.pdf Buxton ILO Chapter 2 Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollmann BC, eds) 14e McGraw-Hill 2023. Katzung, BG Introduction: Chapter 1 The Nature of Drugs & Drug Development & Regulation in Basic and Clinical Pharmacology (Katzung BG Vanderah TW, eds) 15e McGraw Hill 2021. Baca QJ Golan DE Chapter 3 Pharmacokinetics in Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy (Golan DE Armstrons EJ Armstong AW, eds) 4e Wolters Kluwer 2017. Burchum JR Rosenthal LD Charles C Chapter 4 Pharmacokinetics Lehne's Pharmacology for Nursing Care 11e Elsevier 2022. Morris ME Morse BL Chapter 4 Membrane Drug Transporters in Foye's Principles of Medicinal Chemistry (Roche VF Zito SW Lemke TL Williams DA, eds) 8e Wolters Kluwer 2020. Unwin J Jones C Are Drugs Becoming More Lipophilic Over Time? Drug Hunter November 18, 2022 https://drughunter.com/are-drugs-becoming-more-lipophilic-over-time/ Bhal SK Lipophilicity Descriptors: Understanding When to Use LogP & LogD. ACD/Labs Application Note: https://www.acdlabs.com/wp-content/uploads/download/app/physchem/logp_vs_logd.pdf; ACD Labs |