|
|
|
|
|
|
-
-
Pharmacokinetics is described by four processes9
-
Absorption
-
Distribution
-
Metabolism
-
Excretion
-
Absorption describes a drug's movement from the site of administration into the blood.
-
Distribution represents drug translocation from the blood into tissues and ultimately cells.
-
Biotransformation (metabolism) involves a change, usually catalyzed by enzymes, in the parent drug structure which may result in a conversion of an initially inactive "drug" or "pro-drug" to the active form or more commonly, result in formation of parent drug metabolites which may or may no exhibit biological activity.
-
Excretion describes movement of drugs, including metabolites, out of the body.
-
The term elimination represents metabolism plus excretion.
-
These processes taken together affect the drug concentration at the receptor(s).9
-
-
-
-
-
-
-
Biological Membranes and their Importance in Pharmacology: Audio Overview (2025)
-
Movement of drugs from the site of administration to the site/sites of action require movement across cell membranes.
-
Lipophilic and Hydrophilic Drugs
-
Attribution:
-
AMBOSS: Medical Knowledge Distilled
-
Pharmacokinetics-Part 2: Lipophilic and Hydrophilic Drugs 9/27/2019
-
AMBOSS: Medical Knowledge Distilled Youtube Channel:
-
-
-
 Biological membranes consist of phospholipid bilayers (double
sheet).11
Biological membranes consist of phospholipid bilayers (double
sheet).11
-
Lipid-Globular Protein Mosaic Cell Membrane Model10 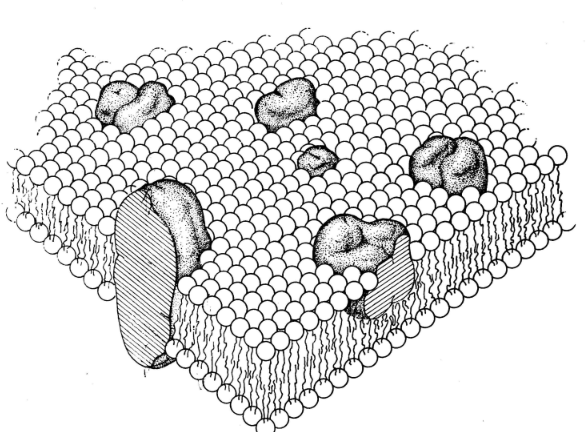
-
Attribution: Figure 3 from Singer SJ Nicolson GL The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972 Feb 18; 175(4023): 720-731.
-
-
The cell membrane is the most important barrier for drug permeation due to the many lipid barriers separating body compartments.
-
Lipid : aqueous drug partition coefficients described the ease with which a drug moves between aqueous and lipid environments.
-
Ionization state of the drug is an important factor: charged drugs diffuse-through lipid environments with difficulty.
-
pH and the drug pKa, important in determining the ionization state, will influence significantly transport.
-
The pH and drug pKa determine the ratio of lipid-to aqueous-soluble forms for weak acids and bases as described by the Henderson-Hasselbalch equation.
-
Uncharged form: lipid-soluble
-
Charged form: aqueous-soluble, relatively lipid-insoluble (does not pass biological membranes easily)
-
-
-
Henderson-Hasselbalch Equation and in Pharmacology: Audio Overview (2025)
-
Henderson-Hasselbalch equation: General form: log (protonated)/(unprotonated) = pKa-pH
-
For Acids: pKa = pH + log (concentration [HA] unionized)/concentration [A-]
-
note that if [A-] = [HA] then pKa = pH + log (1) or (since log(1) = 0), pKa = pH
-
-
For Bases: pKa = pH + log (concentration [BH+] ionized)/concentration [B]
-
note that if [B] = [BH+] then pKa = pH + log (1) or (since log(1) = 0), pKa = pH
-
-
The lower the pH relative to the pKa the greater fraction of protonated drug is found.
-
Recall that the protonated form of an acid is uncharged (neutral); however, protonated form of a base will be charged.
-
-
As a result, a weak acid at acid pH will be more lipid-soluble because it is uncharged and uncharged molecules move more readily through a lipid (nonpolar) environment, like the cell membrane, compared to charged molecules
-
Similarly a weak base at alkaline pH will be more lipid-soluble because at alkaline pH a proton will dissociate from molecule leaving it uncharged and thus free to move through lipid membrane structures
-
Drug Absorption
-
The ionization state of a drug significantly affects its ability to cross biological membranes.
-
Non-ionized (lipophilic) forms of drugs can readily pass through lipid membranes, while ionized (hydrophilic) forms cannot.
-
The Henderson-Hasselbalch equation helps predict the proportion of drug in each form at different pH values throughout the body.
-
-
For example, aspirin (a weak acid with pKa ~3.5) is predominantly non-ionized in the acidic stomach (pH ~2), facilitating absorption.
-
In the small intestine (pH ~6-7), it becomes more ionized, but the large surface area still allows significant absorption.
-
-
-
Ion Trapping
-
Different body compartments have different pH values:
-
Stomach: pH 1-3
-
Small intestine: pH 6-7
-
Blood: pH 7.4
-
Urine: pH 4.5-8
-
-
 These
pH differences result in "ion
trapping."
These
pH differences result in "ion
trapping." -
Weak bases tend to accumulate in acidic compartments (like the stomach), while weak acids accumulate in basic compartments.
-
This principle is used therapeutically and in treating drug overdoses.
-
-
Ion Trapping and Drug Distribution: Audio Overview (2025)
-
-
Drug Distribution
-
The equation helps predict drug distribution across membranes with pH gradients.
-
For instance, local anesthetics (weak bases) can become trapped in infected tissues (which are often acidic), reducing their effectiveness.
-
-
-
Renal Elimination
-
 Urine pH manipulation can enhance
drug elimination.
Urine pH manipulation can enhance
drug elimination. -
Alkalinizing urine (making it more basic) increases excretion of weak acids like barbiturates and aspirin by increasing their ionization.
-
Acidifying urine enhances elimination of weak bases like amphetamines.
-
-
-
-
-
A variety of lipids occur in biological membranes and other components of the membrane include specialized proteins and sugars.11
-
Three major types of lipids are associated with biological membranes: glycolipids, phospholipids, and sterols.
-
Glycolipid (left) and Sterol (right) Structure11 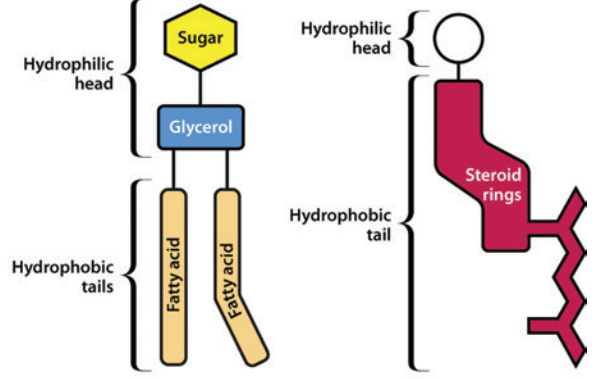
-
Left: Glycolipid
-
Right: Sterol
-
Attribution: Figure 1 (B & C) from reference 11 (Watson H Biological membranes Essays Biochem (2015) 59, 43-70. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626904/pdf/bse0590043.pdf
-
-
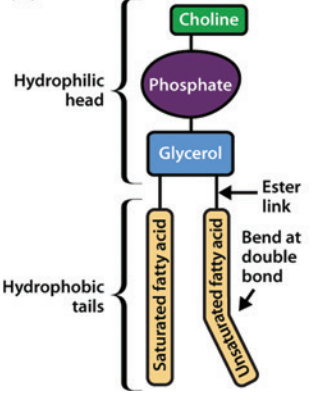
Phospholipids involve two fatty acid chains linked to glycerol and a phosphate group.
-
Those phospholipids which contain glycerol are glycerophospholipids, such as phosphatidylcholine.11
-
Phosphatidylcholine Structure11
-
Phosphatidylcholine
-
Attribution: Figure 1 (A) from reference 11 (Watson H Biological membranes Essays Biochem (2015) 59, 43-70.
-
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626904/pdf/bse0590043.pdf
-
-
-
-
-
![]()
-
Drug absorption generally describes the movement of drug from the site of administration to a central compartment.
-
The free drug (unbound to plasma protein) and localized in the central compartment, such as in the blood, can move to the therapeutic site of action or to tissue reservoirs or become biotransformed (metabolized) and excreted. As illustrated below there are numerous possibilities.
-
Possible Free Drug Movements to and from a Central Compartment13 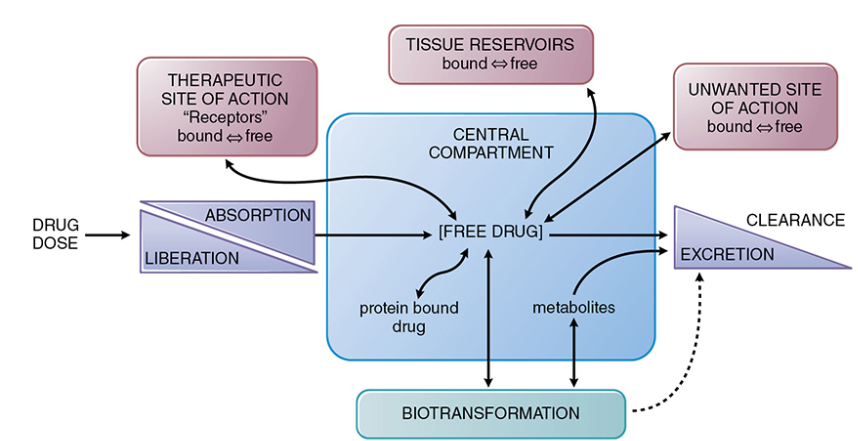
-
Attribution: Figure 2-1 from reference 13 (Buxton ILO Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollmann BC, eds) 14e McGraw-Hill 2023.)
-
-
-
-
For oral administration, absorption follows dissolution of the tablet with elaboration of the drug.13
-
 A number of
factors may influence the rate or extent of dissolution;
however, in the clinical setting the drug's bioavailability
is the more pertinent consideration.
A number of
factors may influence the rate or extent of dissolution;
however, in the clinical setting the drug's bioavailability
is the more pertinent consideration.
-
Bioavailability:
-
Bioavailability represents the fraction of an administered drug dose that reaches the site of drug action or reaches a "biological fluid", typically systemic circulation.13
-
For orally administered drugs, initial significant absorption is from the gastrointestinal tract.
-
However, the extent of absorption is influenced and could be reduced by several factors including:
-
Dosage form
-
Drug's chemical and physical properties
-
Intestinal metabolism of the drug
-
Transport across the intestinal epithelium and into the hepatic portal circulation.
-
First-Pass Effect: When a drug is transported across the intestinal epithelium and enters the hepatic portal circulation, the drug is subjected to potential metabolism and biliary excretion prior to entering the systemic circulation.
-
First-Pass Metabolism and Oral Drug Administration: Audio Overview (2025) -
 Sometimes, the drug may be subject to extensive liver metabolism
or biliary excretion such that bioavailability is decreased
significantly.
Sometimes, the drug may be subject to extensive liver metabolism
or biliary excretion such that bioavailability is decreased
significantly.
-
This scenario is the first-pass effect."13
-
First Pass Metabolism -
Attribution: Saffarzadeh A First Pass Metabolism - Pharmacology Lecture 6
-
https://www.youtube.com/watch?v=5AB8WkCbz4k 9/19/2012.
-
-
-
Note that the first-pass effect supposes oral administration since IV administration allows the total drug dose access to the systemic circulation immediately.13
-
-
-
-
-
-
Following oral administration, there may be incomplete absorption as is noted with the drug digoxin in which only about 60% of a dose will reach systemic circulation.12
-
Incomplete absorption is most often due to reduced absorption in the gut.
-
Recalling that biological membranes are composed of a bilayer in which the central component is lipid, drugs that are "hydrophilic" will have difficulty diffusing through the "hydrophobic" lipid region of the bilayer.
-
By contrast, a drug that is lipophilic might be insufficiently soluble in water to traverse the water layer near the cell.12
-
-
Mucous membranes themselves are protected by several mechanisms including:14
-
Mucociliary clearance in the trachea
-
Lacrimal duct lysozyme secretion
-
Stomach acid
-
Base in the duodenum
-
These "defense mechanisms" albeit nonspecific, represent potential drug absorption barriers and may contribute to reduced drug bioavailability at the intended therapeutic target.14
-
-
-
-
-
Extractions Ratios and Bioavailability12 -
Drug Extraction Ratios in Pharmacology: Audio Overview (2025) -
The magnitude of a first-pass the fact is described by the extraction ratio (ER) which has the form of:
ER = CLliver / Q-
where CL liver represents hepatic blood clearance and Q is the hepatic blood flow.
-
Hepatic blood flow is approximately 90 L/hour (70 kg person).12
-
-
Using this information systemic bioavailability of the drug can be estimated by the extent of absorption (f) and the extraction ratio (ER).
-
The equation for this relationship is:
F = f x (1 - ER).12
-
-
For some drugs, morphine as an example, absorption is nearly complete so f is about equal to 1.
-
The hepatic extraction ratio for morphine is the morphine blood clearance divided by hepatic blood flow or:
-
(60 L/hour/70 kg) / (90 L/hour/70 kg) = 0.67.
-
Therefore, oral bioavailability (1 - ER) is about 33%.12
-
-
Attribution: Reference 12
-
Holford NHG Chapter 3 Pharmacokinetics & Pharmacodynamics: Rational Dosing & the Time Course of Drug Action in Basic and Clinical Pharmacology (Katzung BG Vanderah TW, eds) 15e McGraw Hill 2021.
-
-
-
Routes of Administration and First-Pass Effect Overview12
-
There are numerous routes of drug administration used clinically.
-
Some of these include:
-
Oral administration
-
Topical application such as transdermal
-
Sublingual, and
-
Rectal.
-
-
Hepatic first-pass effects is limited by using sublingual and transdermal routes of administration and reduced by use of rectal suppositories.
-
The sublingual and transdermal routes of administration allow direct access to systemic veins.
-
Following rectal administration, the drug has access above the rectum to veins leading to the liver.
-
As a consequence, approximately only half a rectal dose likely bypasses hepatic metabolism.
-
-
-
Pulmonary: If the drug is administered by inhalation, the hepatic first-pass effect is eliminated but there may be first-pass "loss" by excretion.12
-
-
-
-
Routes of Drug Administration: Audio Overiew (2025)
-
-
 The most
common route of drug administration is by oral ingestion.12
The most
common route of drug administration is by oral ingestion.12
-
Oral administration remains the most common and convenient route for drug delivery, accounting for approximately 60% of all pharmaceutical preparations. 19
-
When drugs are taken orally, they must first dissolve in gastrointestinal fluids before absorption can occur.
-
The primary site of absorption is the small intestine due to its large surface area, estimated at 200-300 squaremeters, and rich blood supply.20
-
-
Several factors influence oral drug absorption.
-
The pH environment varies significantly throughout the gastrointestinal tract, ranging from highly acidic (pH 1-3) in the stomach to more alkaline conditions (pH 7-8) in the small intestine.
-
This pH variation affects drug ionization and subsequently their absorption, as described by the Henderson-Hasselbalch equation. 21
-
Lipophilic drugs generally exhibit better absorption than hydrophilic compounds due to their ability to cross lipid cell membranes more readily.
-
-
-
First-pass metabolism represents a major limitation of oral administration.22
-
After absorption from the gastrointestinal tract, drugs enter the hepatic portal circulation and pass through the liver before reaching systemic circulation.
-
This hepatic first-pass effect may significantly reduce bioavailability, with some drugs undergoing extensive first-pass metabolism before reaching their target sites. 23
-
Additionally, drug-food interactions can alter absorption rates and extent, necessitating specific administration instructions relative to meals.24
-
-
-
-
Controlled-Release Preparations
-
Controlled-release formulations have revolutionized oral drug therapy by maintaining therapeutic drug concentrations over extended periods while reducing dosing frequency.26
-
These preparations employ various mechanisms to control drug release rates, including matrix tablets, and reservoir systems,25
-
Matrix tablets incorporate drugs within polymer networks that control release through diffusion and erosion mechanisms.
-
Hydrophilic matrices, such as those containing hydroxypropyl methylcellulose (HPMC), form gel layers upon contact with aqueous media, creating barriers that modulate drug release.27
-
The Higuchi equation often describes drug release from these systems, where release rate is proportional to the square root of time.28
-
-
-
Reservoir systems encapsulate drugs within polymer membranes that control release rates through membrane permeability. 29
-
These systems can achieve zero-order release kinetics, providing constant drug delivery rates independent of time. 30
-
-
Osmotic pump systems, exemplified by the OROS technology, utilize osmotic pressure gradients to deliver drugs at predetermined rates through laser-drilled orifices. 31
-
-
-
-
-
Sublingual administration involves placing medications under the tongue, where the medication both dissolves and is available for direct absorption through the oral mucosa into the venous circulation. 32
-
This route bypasses hepatic first-pass metabolism and provides rapid onset of action.20
-
Nitroglycerin is the frequently cited example of sublingual administration, typically used to rapidly relieve anginal symptoms.33
-
Predisposing to adequate sublingual bioavailability is nitroglycerin's high lipid solubility which promotes movement through biological membranes.34
-
-
-
-
-
Parenteral routes bypass the gastrointestinal tract entirely, offering advantages including rapid onset, predictable bioavailability, and suitability for unconscious or uncooperative patients.35
-
-
-
Intravenous (IV) administration allows drugs to be delivered directly into the systemic circulation.
-
IV administration allows for 100% bioavailability as well as rapid onset of drug action.
-
This route allows precise control over drug plasma concentrations through adjustment of infusion rates.36
-
-
IV administration is particularly important for drugs with poor oral bioavailability, such as aminoglycosides (e.g. digoxin, digitoxin).37
-
Continuous IV infusions maintain steady-state plasma concentrations, following the equation: Css = k0/CL, where Css represents steady-state drug concentration, k0 is the infusion rate constant, and CL is clearance.38
-
 IV
administration carries risks including phlebitis, infiltration,
and potential for rapid adverse reactions due to immediate
systemic exposure.39
IV
administration carries risks including phlebitis, infiltration,
and potential for rapid adverse reactions due to immediate
systemic exposure.39
-
-
-
-
-
-
Subcutaneous (SC) injection delivers drugs into the loose connective tissue beneath the dermis.
-
This route provides slower, more sustained absorption compared to intramuscular injection, with drugs reaching systemic circulation through capillary networks or lymphatic drainage. 40
-
-
-
Intramuscular Administration41
-
Drug absorption by the intramuscular route of administration may be rapid, given dependence on blood flow rate at the injection site.
-
Rate of absorption may be altered by local heating, exercise or regional massage.
-
Drug absorption tends to be higher if in aqueous drug preparation is injected into the deltoid or vastus lateralis muscle compared to injections in the.
-
This difference is likely associated with the higher fat content, given that fat is comparatively more slowly perfused by blood.
-
-
By contrast of the drug is injected in an oil solution, absorption from the intramuscular site is likely to be slow and constant. 41
-
-
-
Intrathecal Administration41, 42
-
The blood-brain barrier (BBB) and the blood-CSF barrier tend to limit or even prevent drug access to the CNS.
-
This result is due to action of certain membrane transporters such as P-glycoprotein (MDR1) that transport drugs from the central nervous system.
-
If drug effects are required on the meninges or cerebrospinal axis (e.g. spinal anesthesia) drugs may be directly injected into the spinal subarachnoid space.
-
Similarly brain tumors or CNS infections may also be amenable by direct intraventricular drug administration.
-
Intrathecal Chemotherapy
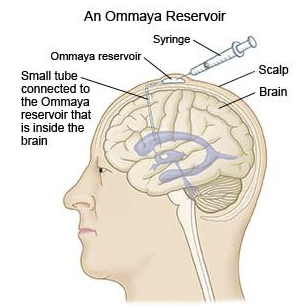
-
Attribution:
-
OpenStax, CC BY 4.0 <https://creativecommons.org/licenses/by/4.0>, via Wikimedia Commons
-
Intrathecal Chemotherapy (last updated on June 2, 2025) Medically reviewed by Drugs.com
-
-
-
-
-
-
-
Pulmonary Absorption: Audio Overiew (2025)
-
Pulmonary drug absorption represents an important route of administration that leverages the unique anatomical and physiological characteristics of the respiratory system for both local and systemic drug delivery.
-
The lungs offer several advantages for drug absorption:
-
Large surface area of approximately 50-75 square meters 43
-
Extensive vascularization, and
-
Thin alveolar-capillary barrier of only 0.1-0.2 micrometers. 44
-
-
-
The respiratory tract can be divided into two main regions with distinct absorption characteristics.
-
The conducting airways, comprising the trachea, bronchi, and bronchioles, have a relatively thick epithelium with ciliated cells and mucus production that can impede drug absorption. 45,46
-
In contrast, the alveolar region consists of a thin epithelium composed primarily of type I and type II pneumocytes, facilitating rapid drug transfer. 46,47
-
"Cross-section of an alveolus and capillary showing diffusion of gases" 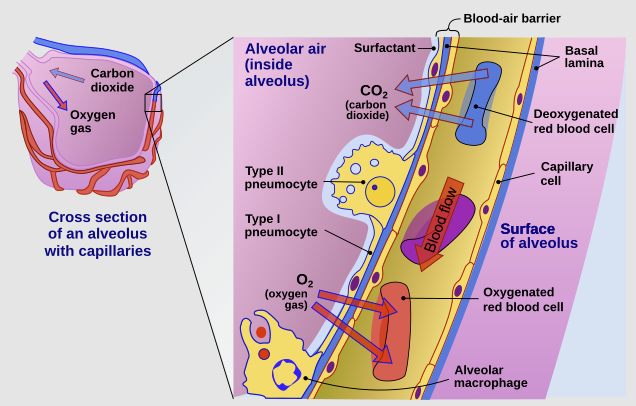
-
Attribution
-
"The cross-section of an alveolus with capillaries is shown. Part of the cross-section is magnified to show diffusion of oxygen gas and carbon dioxide through Type I pneumocytes and capillary cells."
-
Delmalani18, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
-
https://commons.wikimedia.org/wiki/File:Cross_section_of_an_alveolus_and_capillaries_showing_diffusion_of_gases.svg
-
-
-
-
-
-
The pulmonary circulation receives the entire cardiac output, approximately 5 liters per minute, providing excellent perfusion for systemic drug absorption.48
-
The lungs bypass first-pass hepatic metabolism, potentially increasing bioavailability compared to oral administration, however, first-pass effect may also occur in the lungs (as well as other metabolically active tissues).49
-
-
Mechanisms of Pulmonary Drug Absorption
-
Passive diffusion: 45,46
-
This is the principal mechanism for absorption of many drugs.
-
Lipophilic drugs are primarily absorbed by this passive diffusion mechanism.
-
By contrast, hydrophilic drugs are absorbed by diffusion through tight junctions.
-
Of the two, passive diffusion usually results in faster absorption through respiratory epithelium into the bloodstream compared to the latter.46
-
-
Passive diffusion describes movement of drugs across the cell membrane from or region of relatively higher concentration to region of lower concentration.
-
The specific mechanism of absorption for macromolecules in this setting remains to be fully elucidated but may involve movement of drugs through the cells by means of absorptive transcytosis (adsorptive or receptor-mediated), paracellular transport between bijunctions or trijunctions, or by way of large transitory epithelial pores due to cell injury or apoptosis.45
-
-
-
Paracellular Transport:50
-
Small, water-soluble drugs can pass through the tight junctions between epithelial cells.
-
The permeability of these junctions can sometimes be influenced by additives (excipients) which may improve bioavailability.
-
-
-
Carrier-Mediated Transport:
-
Some drugs may utilize specific transporter proteins to cross the epithelial barrier.
-
This mechanism can be either active (requiring energy) or facilitated.51
-
-
-
Endocytosis: 52
-
Peptides and proteins, may be engulfed by the cell membrane and transported across the cell in vesicles.
-
-
-
Factors Affecting Pulmonary Drug Absorption
-
Particle Size:45
-
This is an important factor.
-
For a drug to reach the deep lung, the optimal aerodynamic particle size is between 1 and 5 micrometers.
-
Larger particles tend to deposit in the upper airways and are cleared by swallowing, while smaller particles may be exhaled.
-
-
-
For example, macromolecules in the range of < 5-6 nanometers, following inhalation into the long rapidly appear in the systemic circulation.
-
Insulin with the diameter of 2.2 nm requires about 15-60 minutes after inhalation to attain peak blood levels.
-
Macromolecules >5-6 nm in diameter exhibits low pulmonary absorption, taking many hours.45
-
-
-
Breathing Pattern:
-
A slow, deep inhalation followed by a period of breath-holding enhances the deposition of drug particles in the distal airways and alveoli, allowing more time for absorption.53
-
-
Physicochemical Properties of the Drug:54
-
Factors such as molecular weight, lipophilicity, and solubility influence how a drug is absorbed.
-
For instance, more lipophilic drugs tend to be absorbed more rapidly via passive diffusion.
-
-
-
Inhalation Device: 53,55
-
The type of inhaler used, such as a metered-dose inhaler (MDI), dry powder inhaler (DPI), or nebulizer, significantly impacts the particle size distribution and the efficiency of drug delivery to the lungs.
-
-
Disease State:
-
Conditions like asthma or COPD can alter the airway geometry, increase mucus production, and affect the distribution and absorption of inhaled drugs.56
-
-
-
Advantages of Pulmonary Drug Administration45
-
Rapid Onset of Action:
-
Direct delivery to the large and highly vascularized surface of the lungs allows for quick absorption and a fast therapeutic effect, which is crucial for rescue medications in asthma.
-
-
High Bioavailability:
-
By avoiding the gastrointestinal tract and first-pass metabolism in the liver, drugs administered through the lungs can achieve higher bioavailability compared to oral administration (Although first-pass metabolism may occur in the lungs as well as other metabolically active tissues).
-
-
Lower Doses and Reduced Systemic Side Effects:
-
For treating lung diseases, direct application allows for the use of smaller doses, which can minimize adverse effects on other parts of the body.
-
" Advantages of Pulmonary Delivery of Drugs to Treat Respiratory and Systemic Disease" Treatment of respiratory diseases Treatment of systemic diseases -
Delivery of the drug directly to the disease site
-
Minimize risk of systemic side effects
-
Fast clinical response
-
Bypass the various to therapeutic efficacy, such as poor gastrointestinal absorption and first-pass hepatic metabolism.
-
Achieve a similar or enhanced therapeutic effect at a much reduced dose, compared to a systemic dose. (Salbutamol administered at an oral dose of 2-4 mg is equivalent to 100-200 µg administered by a metered-dose inhaler (MDI).
-
Noninvasive delivery system (no needles)
-
Appropriate for a wide range of drugs
-
Large surface area for drug absorption (about 100 m2) along with high membrane permeability in the alveolar region.
-
Large molecules exhibiting very low rates of absorption may allow substantial amounts of drug absorption.
-
Slow mucociliary clearance in the long periphery promotes extended presence of the drug in the lung.
-
-
Possible first-pass metabolism in the lung is much much less than first-pass liver metabolism.
-
Reproducible absorption kinetics in which delivery is independent dietary complications, extracelluar enzymes or interpatient metabolic differences that influence drug absorption in the gastrointestinal tract.
-
Attribution:
-
Adapted from Table 1 Reference 45- Advantages of pulmonary delivery of drugs to treat respiratory and systemic disease.
-
Primary reference 45 which contains additional reference to some specific information in the table.
-
Reference 45: Labiris N Dolovich M Pulmonary drug delivery. Part 1: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J Clin Pharmacol. 2003 December; 56(610 ): 588-599. https://pmc.ncbi.nlm.nih.gov/articles/PMC1884307/
-
-
-
-
-
Some Examples of Inhaled Drugs
-
Bronchodilators e.g. albuterol and salmeterol
-
Corticosteroids e.g. fluticasone and budesonide
-
Antibiotics e.g. tobramycin
-
Systemic Drugs e.g. inhaled insulin (Alfrezza)
-
-
-
-
Absorption across mucous membranes
-
Topical administration of drugs to mucous membranes represents a versatile and often advantageous route for both local and systemic therapies.
-
These specialized tissues, lining cavities such as the mouth, nose, rectum, and vagina, offer a unique interface for drug absorption, bypassing some of the hurdles associated with oral ingestion.
-
This method of delivery can provide rapid onset of action, targeted treatment, and improved bioavailability for a wide range of medications.
-
-
-
Mucus membranes
-
Mucous membranes, or mucosae, are characterized by a surface layer of epithelial cells, an underlying connective tissue layer (lamina propria), and a thin layer of smooth muscle (muscularis mucosae).57
-
These membranes are kept moist by the secretion of mucus, a viscoelastic fluid that plays a protective and lubricative role. 58
-
The high vascularity of the submucosal region and the relatively thin nature of the epithelial barrier in many mucosal tissues make them permeable to various drug molecules.59
-
These characteristics allow for direct absorption into the systemic circulation, circumventing the harsh environment of the gastrointestinal (GI) tract and the first-pass metabolism in the liver, where a significant portion of an orally ingested drug can be inactivated before it reaches the bloodstream.59
-
-
-
Sites of Mucosal Drug Delivery
-
Oral mucosa (Buccal and Sublingual)
-
The oral cavity presents two primary routes for mucosal drug delivery: the buccal mucosa (the lining of the cheek) and the sublingual mucosa (the area under the tongue). 60
-
The sublingual route, with its thin, highly vascularized epithelium, is particularly well-suited for rapid absorption and is often used for drugs requiring a quick onset of action, such as nitroglycerin for angina attacks. 61
-
The buccal mucosa offers a larger, less permeable surface suitable for sustained-release formulations.62
-
-
Both oral mucosal routes avoid destructive acidic and enzymatic environment of the stomach.
-
-
Nasal Mucosa
-
 The nasal cavity
boasts a large, highly vascularized surface area and a relatively
permeable epithelium, making it an excellent site for rapid systemic
drug delivery.63
The nasal cavity
boasts a large, highly vascularized surface area and a relatively
permeable epithelium, making it an excellent site for rapid systemic
drug delivery.63 -
This route is not only used for local treatments like decongestants but also for systemic medications, including migraine medications and vaccines.64
-
Nasal mucosa provides a potential direct pathway to the central nervous system, offering a non-invasive approach for delivering drugs to the brain, bypassing the blood-brain barrier.65
-
-
-
Vaginal and Rectal Mucosa
-
Vaginal and rectal routes are effective for both local and systemic drug administration.68
-
Vaginal mucosa is a common site for delivering treatments for local infections and for hormonal therapies.20
-
 The rectal mucosa
has a rich blood supply and can be used for systemic drug delivery,
especially when oral administration is not feasible due to nausea,
vomiting, or unconsciousness.67
The rectal mucosa
has a rich blood supply and can be used for systemic drug delivery,
especially when oral administration is not feasible due to nausea,
vomiting, or unconsciousness.67 -
Venous drainage from the lower rectum partially bypasses the liver, reducing first-pass metabolism.66
-
"Schematic showing venous and lymphatic drainage from the rectum and portosystemic shunting" 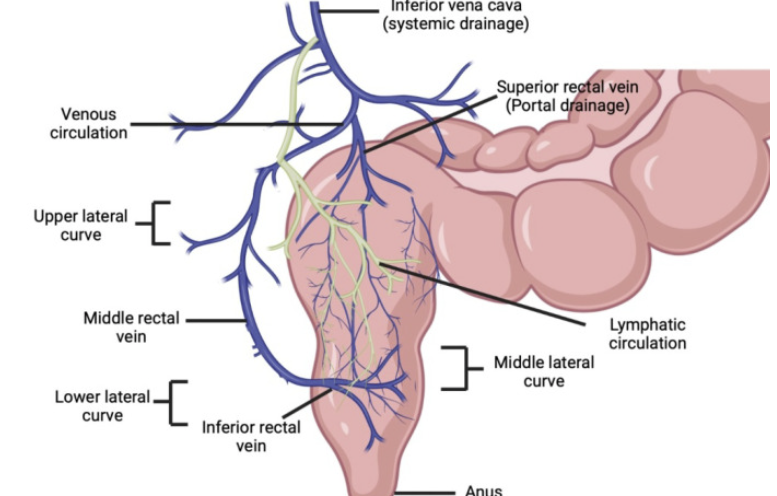
-
Attribution:
-
Rathi R Sanshita Kumar A Vishvakarma V Huanbutta K Singh I Sangnim T Advances in Rectal Drug Delivery Systems: Clinical Trials, and Patients Perspective. Pharmaceutics. 2022 October 17;14(10): 2210. https://pmc.ncbi.nlm.nih.gov/articles/PMC9609333/ Reference 67.
-
-
-
-
-
-
-
-
-
Ophthalmic Drug Administration: Audio Overview
Introduction:
-
Topical ophthalmic medications represent the primary route of drug administration for treating anterior segment eye diseases and some posterior segment conditions.69
-
Topical administration represents the preferred method for many ocular conditions due to its non-invasive nature and direct application to the site of action and importantly patient compliance.70
-
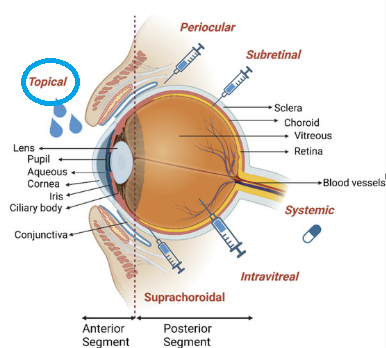
-
Attribution
-
"Anatomy of the eye
-
Anatomy and posteriorly I is given with the major corresponding tissues labeled.
-
"Main drug administration routes to the eye including topical, suprachoroidal, intravitreal, subretinal, periocular, and systemic delivery are shown." Illustrations were created using the Biorender with agreement number as specified in the original reference (70).
-
-
Bal-Ozturk A Ozcan-Bulbui E Gulekin H Cecen B Demir E Zarepour A Cetinel S Zarrabi A Application of Converging Science and Technology toward Ocular Disease Treatment. Pharmaceuticals 2023, 16(3), 445. https://www.mdpi.com/1424-8247/16/3/445
-
-
-
Anatomical and Physiological Considerations
-
The cornea serves as the primary barrier to drug penetration into the eye, consisting of three main layers: the lipophilic epithelium, hydrophilic stroma, and lipophilic endothelium.71
-
This alternating lipophilic-hydrophilic structure results in particular pathways for lipophilic and hydrophilic drugs respectively.72
-
-
The conjunctiva and sclera provide alternative routes for drug absorption, particularly for hydrophilic compounds that poorly penetrate the corneal epithelium.
-
However, systemic absorption through conjunctival and nasal blood vessels can reduce local bioavailability and potentially cause systemic side effects.73
-
Ophthalmic Drug Classifications
-
Anti-Infective Drugs
-
Antibacterial agents are commonly used topical ophthalmic medications.
-
Fluoroquinolones, such as moxifloxacin and gatifloxacin, have become first-line treatments for bacterial conjunctivitis and keratitis due to their broad spectrum of activity and excellent ocular penetration.74
-
Aminoglycosides, including tobramycin and gentamicin, remain important for treating Pseudomonas aeruginosa infections despite concerns about corneal toxicity with prolonged use.75
-
-
Antiviral medications
-
Antiviral agents like trifluridine and ganciclovir gel are primarily used for treating herpes simplex keratitis.76
-
Antifungal therapy options remain limited, with natamycin suspension being the only commercially available topical antifungal in many countries, though compounded preparations of voriconazole and amphotericin B are increasingly used for severe fungal keratitis.77
-
-
-
Anti-Inflammatory Drugs
-
Corticosteroids remain the cornerstone of anti-inflammatory therapy in ophthalmology.
-
Prednisolone acetate 1% provides excellent anti-inflammatory activity for anterior uveitis and post-surgical inflammation.79
-
Newer formulations like loteprednol etabonate offer reduced risk of intraocular pressure elevation through rapid metabolism to inactive compounds.80
-
Difluprednate, a potent difluorinated corticosteroid, allows for less frequent dosing due to enhanced penetration and potency.79
-
-
Nonsteroidal anti-inflammatory drugs (NSAIDs)
-
Non-steroidal anti-inflammatory drugs (NSAIDs) such as ketorolac, bromfenac, and nepafenac are valuable for managing post-operative inflammation and cystoid macular edema.81
-
These drugs work by inhibiting cyclooxygenase enzymes, reducing prostaglandin synthesis without the steroid-associated risks of elevated intraocular pressure or cataract formation.82
-
-
-
-
Glaucoma Medications
-
The management of glaucoma has been revolutionized by prostaglandin analogs, including latanoprost, travoprost, and bimatoprost, which increase uveoscleral outflow and provide once-daily dosing.83
-
β-blockers like timolol remain important second-line agents, though systemic absorption can cause cardiovascular and respiratory side effects.84,85
-
-
α-2 agonists such as brimonidine provide dual mechanisms by decreasing aqueous production and increasing uveoscleral outflow.88
-
Alpha2 Agonists Useful in Glaucoma Management 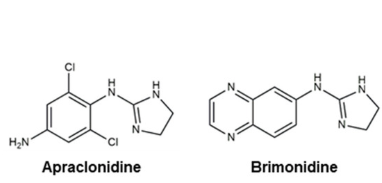
-
-
Carbonic anhydrase inhibitors, available as dorzolamide and brinzolamide, reduce aqueous humor production and can be used as monotherapy or in fixed combinations.89 .
-
The newest class, rho kinase inhibitors like netarsudil, work by increasing trabecular outflow and represent a novel mechanism for IOP reduction.90,91 .
-
Rho kinase inhibitors a.k.a. ROCK inhibitor are compounds targeting Rho kinase and inhibit ROCK the pathway.
-
Although recently Rho kinase inhibitors have been evaluated for glaucoma therapy, these agents may be useful in management of cardiovascular diseases including ischemic stroke.92
-
-
-
-
Mydriatics and Cycloplegics
-
Tropicamide provides short-acting mydriasis useful for diagnostic procedures, while cyclopentolate offers intermediate duration of action for both mydriasis and cycloplegia.86
-
Atropine, the longest-acting agent, is experiencing renewed interest for myopia control in children, with low-concentration formulations (0.01-0.05%) showing promise in slowing myopic progression.87
-
-
Drug-eluting stents (DES) emerged as a important solution to the problem of in-stent restenosis following percutaneous coronary intervention. 93
-
These devices combine mechanical scaffolding with localized pharmacotherapy, delivering antiproliferative agents directly to the arterial wall to prevent smooth muscle cell proliferation and neointimal hyperplasia.94
-
Some Antiproliferative Drugs used to prevent Restenosis following Stenting 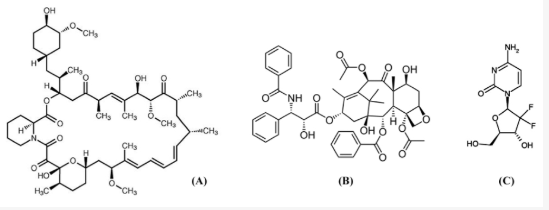
-
Attribution
-
"Chemical structures of antiproliferative drugs: sirolimus (A) ; paclitaxel (B); and gemcitabine (C).
-
Corresponds to figure 1 in: Corresponds to figure 1 in: Kwon H Park Local Delivery of Antiproliferative Agents via Stents. Polymers 2014, 6(3), 755-775. https://www.mdpi.com/2073-4360/6/3/755
-
-
-
The first-generation DES utilized sirolimus-eluting stents (Cypher, Cordis) and paclitaxel-eluting stents (Taxus, Boston Scientific). Some studies demonstrated a reduction in restenosis rates.96
-
 Concerns
emerged regarding late stent thrombosis, attributed to delayed
endothelialization and hypersensitivity reactions to the durable polymer
coatings.95
Concerns
emerged regarding late stent thrombosis, attributed to delayed
endothelialization and hypersensitivity reactions to the durable polymer
coatings.95 -
In this study (95) stent thrombosis after one year actually was more common in the drug-eluting stents compared with beer-metal stands.
-
Also, target-lesion revascularization was reduced when drug-eluting stents were employed. At four years, no differences in cumulative death rates or myocardial infarction were reported.
-
-
-
Second-generation drug-eluting stents addressed these limitations through improved stent platforms, biocompatible polymers, and alternative drug formulations.
-
The everolimus-eluting stent (Xience V, Abbott Vascular) and zotarolimus-eluting stent (Endeavor, Medtronic) demonstrated superior safety profiles while maintaining efficacy.97
-
Clinical trials such as SPIRIT IV showed that everolimus-eluting stents significantly reduced target lesion failure compared to paclitaxel-eluting stents at one year (4.2% vs 6.8%, p=0.001).98
-
-
Bone: Tetracycline Accumulation
-
Introduction
-
Tetracyclines are a class of broad-spectrum antibiotics that exhibit a unique property of binding to calcium-containing tissues, particularly bone and teeth.
-
This characteristic has significant implications for both therapeutic applications and potential adverse effects, especially in developing individuals.2B,110
-
-
-
Bone Accumulation: Mechanism
-
Tetracyclines demonstrate high affinity for calcium ions through chelation, forming stable complexes that preferentially deposit in calcifying tissues.111
-
The mechanism involves the binding of tetracycline molecules to hydroxyapatite crystals, the primary mineral component of bone matrix.112
-
This binding occurs through the formation of coordinate bonds between the antibiotic's functional groups and calcium ions within the bone structure.2B
-
The accumulation process is particularly pronounced in areas of active bone formation and remodeling, where newly formed hydroxyapatite provides abundant binding sites for tetracycline molecules.
-
The drug becomes incorporated into the bone matrix during the mineralization process, creating a reservoir that can persist for extended periods.2B,113
-
-
-
-
-
-
Clinical Correlations
-
The bone-seeking properties of tetracyclines have been used therapeutically in certain bone disorders.
-
Low-dose tetracycline therapy has shown efficacy in treating osteoporosis by inhibiting bone resorption and promoting bone formation114 .
-
Tetracyclines anti-inflammatory properties may also benefit patients with inflammatory bone conditions.115
-
-
-
-
Adverse Effects
-
Tetracycline accumulation in bone can lead to several adverse effects, particularly in pediatric populations and pregnant women including:
-
Interfering with skeletal development
-
In children, tetracycline deposition can interfere with normal bone growth and development.110
-
This effect may lead to growth retardation and skeletal abnormalities.116
-
-
Tooth Discoloration and Defects
-
Tetracycline binding to developing tooth structures results in characteristic yellow-brown discoloration and enamel hypoplasia.
-
These effects are particularly pronounced when exposure occurs during tooth development in utero through age 8 years.117,118
-
-
-
-

-
-
Fick's Law of Diffusion: Audio Overview (2025)
-
Fick's Law describes passive movement molecules down its concentration gradient.
Flux (J) (molecules per unit time) = (C1 - C2) · (Area ·Permeability coefficient) / Thickness
-
Where C1 is the higher concentration and C2 is the lower concentration
-
Area = area across which diffusion occurs
-
Permeability coefficient: drug mobility in the diffusion path
-
For lipid diffusion, lipid: aqueous partition coefficient -- major determinant of drug mobility
-
Partition coefficient reflects how easily the drug enters the lipid phase from the aqueous medium.
-
-
-
Thickness: length of the diffusion path
-
Although there certain disadvantages such as limited drug absorption in some cases or emesis due to G.I. irritation, oral administration is considered the most convenient, economical and safest approach.
-
Following oral administration, the drug may be metabolized by enzymes associated with the G.I. microbiome, mucosa or liver prior to gaining systemic access.
-
Many factors influence the absorption of drugs from the gastrointestinal tract.
-
Factors include:
-
Absorption surface area
-
Blood flow to the area of absorption
-
Physical state of the drug (solution, suspension etc.)
-
Aqueous solubility of the drug
-
The drug concentration where absorption occurs.13
-
-
-
 Drug absorption
from the G.I. tract is mainly by passive aqueous diffusion.13
Drug absorption
from the G.I. tract is mainly by passive aqueous diffusion.13
-
The driving force is the drug concentration gradient.
-
Therefore, absorption is more likely when the drug is in the unionized (nonionized) form.
-
Some drugs can be described as either "weak acids" or "weak bases."
-
A weak acid becomes ionized when it loses a positively charged H+; by contrast, a weak base becomes ionized when it accepts a positively charged H+.
-
A acidic drug' s pKa when compared with the pH of the aqueous environment describes the ease by which the drug may lose a proton and become negatively charged.
-
The converse argument applies to drugs which are weak bases.
-
-
-
In the case of drugs defined as weak acids, better absorption would be predicted in a more acidic environment, such as the stomach with a pH range of 1-2.
-
Absorption the same week acid drug would be less likely ionized at the higher pH values (3-6) typical of the upper intestine.
-
The inverse of this description would apply to weak bases.13
-
-
-
Drugs that are weak acids or bases
-
A weak acid is a neutral molecule that dissociates into an anion (negatively charged) and a proton (a hydrogen ion). For example:
-
C8H7O2COOH ⇄ C8H7O2COO- + H+
-
Neutral aspirin (C8H7O2COOH) in equilibrium with aspirin anion (C8H7O2COO- ) and a proton (H+ )
-
Weak acid: protonated form -- neutral, more lipid-soluble
-
-
Weak base: a neutral molecule that can form a cation (positively charged) by combining with a proton. Example:
-
C12H11CIN3NH3+ ⇄ C12H11CIN3NH2 + H+
-
Pyrimethamine cation (C12H11CIN3NH3+) in equilibrium with neutral pyrimethamine (C12H11CIN3NH2) and a proton (H+ )
-
Weak base: protonated form which is charged and therefore less lipid-soluble.
-
-
-
Examples of drugs that are weak acids or weak bases:
Weak acids
pKa
Weak bases
pKa
-
Phenobarbital (Luminal)
7.1
-
Cocaine
8.5
-
Pentobarbital (Nembutal)
8.1
-
Ephedrine
9.6
-
Acetaminophen
9.5
-
Chlordiazepoxide (Librium)
4.6
-
Aspirin
3.5
-
Morphine
7.9
-
-
-
-
-
-
-
"Protective barriers of the brain" 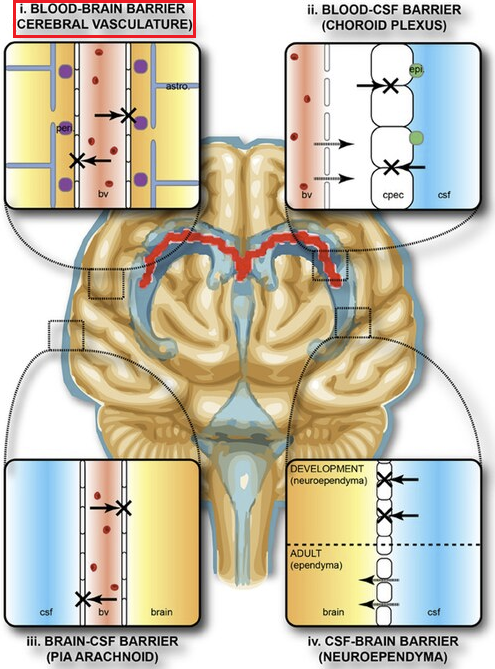
-
Attribution
-
Stolp HB, Liddelow SA, Sá-Pereira I, Dziegielewska KM and Saunders NR, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
-
Protective barriers of the brain (2013-08-23)
-
https://commons.wikimedia.org/wiki/File:Protective_barriers_of_the_brain.jpg
-
-
"Protective areas of the brain. The collective term "blood-brain-barrier" is used to describe four main interfaces between the central nervous system and the periphery.
-
(1) The blood-brain barrier proper formed by tight junctions between the endothelial cells of the cerebral vasculature. It is thought that pericytes (peri) are sufficient to induce some barrier characteristics and endothelial cells, while astrocytes (astro.) are able to maintain the integrity of the blood-brain barrier postnatally."
-
Follow link (https://commons.wikimedia.org/wiki/File:Protective_barriers_of_the_brain.jpg) for complete figure description.
-
-
The delivery of therapeutic agents to the central nervous system (CNS) is a significant challenges in medicine.100
-
The brain and spinal cord are protected by highly selective barriers that, while essential for maintaining neural homeostasis and protecting against pathogens, limit the penetration of potentially beneficial therapeutic compounds.101
-
These barriers prevent the entry of 100% of large-molecule neurotherapeutics and more than 98% of all small-molecule drugs.99
-
Structure and Composition
-
The blood-brain barrier is a highly selective semipermeable border of endothelial cells that regulates the transfer of solutes and chemicals between the circulatory system and the central nervous system.102
-
The BBB is composed of several important components which together form the barrier barrier:
-
Endothelial Cells
-
Brain endothelial cells are considered the BBB's core anatomical structure, differing significantly from peripheral endothelial cells in both morphology and function given that the former have apical type junctional complexes which more closely resemble epithelium than endothelium.103
-
Brain endothelial cells are characterized by:
-
Tight junctions that fasten cells together, creating distinct luminal and abluminal (outer surface)membrane compartments. 104
-
Absence of fenestrations (small transcellular pores), which limits free diffusion between brain tissue and blood. 104
-
-
-
Tight Junctions
-
Tight junctions are the main functional components in sustaining the permeability barrier and controlling tissue homeostasis. 105
-
The major tight junction proteins include
Claudins and occludins localized at two-cell contacts.
-
"Cellular tight junction" 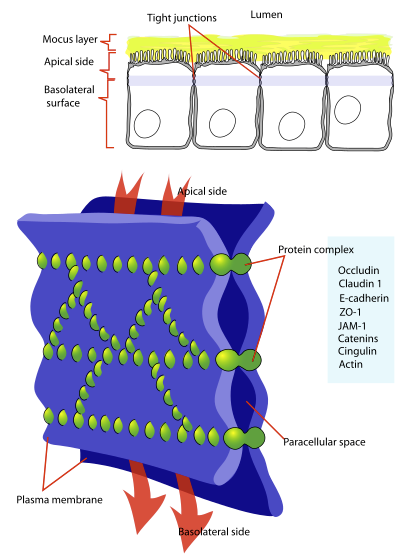
-
Attribution
-
LadyofHats, Public domain, via Wikimedia Commons
-
"Diagram showing a tight junction. Tight junctions seal adjacent epithelial cells in a narrow band just beneath their apical surface."
-
-
https://commons.wikimedia.org/wiki/File:Cellular_tight_junction-en.svg
-
-
-
Tricellulin and lipolysis-stimulated lipoprotein receptor reside at three-cell contacts.105, 106
-
-
-
-
Supporting Cells107
-
Astrocytes:
-
End feet connect with the basement membrane playing roles in dynamic signaling, waste clearing, brain blood flow regulation, and neuroimmune responses.
-
-
Pericytes:
-
Embedded in the basement membrane, these cells are central to neurovascular unit function, communicating with endothelial cells through various signaling pathways.
-
-
-
-
Physiological Functions
-
The BBB maintains brain homeostasis by regulating specific ion channels and transporters.108
-
Ion regulation:
-
Na+, K+, Ca2+, Cl- are maintained at optimal levels for neural and synaptic signaling through asymmetric distribution between luminal and abluminal membranes.
-
-
Selective permeability:
-
The tight gap allows only passive diffusion of lipid-soluble drugs at a molecular weight lower than 400-600 Daltons (Da).
-
-
Active transport:
-
Specialized transporters facilitate the movement of essential molecules like glucose and amino acids while efflux pumps remove unwanted substances.
-
-
-
-
-
-
Barriers to Drug Entry
-
Efflux Transporters
-
The presence of P-glycoprotein (referred to as multidrug resistance associated membrane protein) allows drugs to be transported back into the blood by ATP-dependent efflux pumps.109
-
-
-
-
-
Stomach and Gastrointestinal Tract Absorption
-
The stomach surface area supporting drug absorption is comparatively small, estimated at about 500 cm2. 16
-
The upper intestine villi is associated with the much larger surface area estimated to be in the range of 32 m2 to 200 m2.16,13
-
 As a consequence
of the substantial difference in surface areas, the drug absorption rate
in the intestine is typically greater compared to the stomach even in
the case in which the drug might be ionized (intestine) and nonionized
(stomach).
As a consequence
of the substantial difference in surface areas, the drug absorption rate
in the intestine is typically greater compared to the stomach even in
the case in which the drug might be ionized (intestine) and nonionized
(stomach).
-
-
Elevated gastric emptying favors increased drug absorption, typically.
-
Gastric emptying refers to stomach emptying with contents moving into the duodenum which depends on peristaltic waves, contraction of the antrum and reduced stomach size.18
-
For most drugs, increased gastric emptying rate and increased gastrointestinal motility promotes enhanced drug absorption.
-
 Exceptions
include digoxin and riboflavin in which elevated G.I. motility decreases
rate of absorption.17
Exceptions
include digoxin and riboflavin in which elevated G.I. motility decreases
rate of absorption.17
-
-
-
Rates of gastric emptying may be altered by many factors.
-
Factors Influencing Rates of Gastric Emptying17 Food
Posture
Hormones
Peritoneal irritation
Severe pain
Diabetes
Narcotic analgesics
Anticholinergics
Antacids
Metochlopramide
Ganglionic blocking agents
Alcohol
-
-
-

References
-
Maher TJ Kiel D Chapter 6 G-Protein-Coupled Receptors in Foye's Principles of Medicinal Chemistry (Roche VF Zito SW Lemke TL Williams DA, eds) 8e Wolters Kluwer 2020.
-
2A: Alenghat FJ Golan DE Chapter 1 Drug-Receptor Interactions in Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy (Golan DE Armstrons EJ Armstong AW, eds) 4e Wolters Kluwer 2017; 2B: Buxton I Chapter 2 Pharmacokinetics: The Dynamics of Drug Absorption, DIstribution, Metabolism, and Elimination In Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollman BC eds) McGraw Hill LLC (2023).
-
von Zastrow M Chapter 2 Drug Receptors & Pharmacodynamics in Basic & Clinical Pharmacology ( Katzung BG Vanderah TW, eds) 14e McGraw Hill 2021.
-
Flood P Shafer SL Chapter 2 Basic principles of Pharmacology in Stoelting's Pharmacology & Physiology Anesthetic Practice (Flood P Rathmell JP Urman RD, eds) 6e 2022.
-
Manning DR Blumenthal DK Chapter 3 Pharmacodynamics: Molecular Mechanisms of Drug Action in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollmann BC, eds) 14e McGraw-Hill 2023.
-
Burchum JR Rosenthal LD Charles C Chapter 5 Pharmacodynamics Lehne's Pharmacology for Nursing Care 11e Elsevier 2022.
-
Katzung, BG Introduction: Chapter 1 The Nature of Drugs & Drug Development & Regulation in Basic and Clinical Pharmacology (Katzung BG Vanderah TW, eds) 15e McGraw Hill 2021.
Geroge Jr AL Neilson EG Chapter 309 Cell Biology and Physiology of the Kidney in Harrison's Principles of Internal Medicine (Loscalzo J Kasper DL Longo DL Fauci AS Hauser SLs Jameson JL, eds) 21e 2022.
Burchum JR Rosenthal LD Charles C Chapter 4 Pharmacokinetics Lehne's Pharmacology for Nursing Care 11e Elsevier 2022.
Singer SJ Nicolson GL The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972 Feb 18; 175(4023): 720-731.
Watson H Biological membranes Essays Biochem (2015) 59, 43-70. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626904/pdf/bse0590043.pdf
Holford NHG Chapter 3 Pharmacokinetics & Pharmacodynamics: Rational Dosing & the Time Course of Drug Action in Basic and Clinical Pharmacology (Katzung BG Vanderah TW, eds) 15e McGraw Hill 2021.
Buxton ILO Chapter 2 Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollmann BC, eds) 14e McGraw-Hill 2023.
Baca QJ Golan DE Chapter 3 Pharmacokinetics in Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy (Golan DE Armstrons EJ Armstong AW, eds) 4e Wolters Kluwer 2017.
Tsunoda SM Dorrestein PC Knight Rob Chapter 6 The Gastrointestinal Microbiome and Drug Response in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollmann BC, eds) 14e McGraw-Hill 2023.
Helander HF Fandriks L Surface are of the digestive tract-revisited Scand J Gasatroenterol. 2024 Jun; 49(6): 683-9 https://pubmed.ncbi.nlm.nih.gov/24694282/ ;Bionumbers (B10Numb3R5); https://bionumbers.hms.harvard.edu/bionumber.aspx?s=n&v=5&id=111126.
Nimmo WS Drugs, diseases and altered gastric emptying Clin Pharmacokinet. 1976;1(3): 189-203. https://pubmed.ncbi.nlm.nih.gov/797497/
Jacoby HI Reference Module in Biomedical Sciences, 1027 https://www.sciencedirect.com/topics/medicine-and-dentistry/stomach-emptying# ; https://www.sciencedirect.com/science/article/abs/pii/B9780128012383649218
Alquhtani M Kazi M Alsenaidy M Ahmad M Advances in Or Drug Delivery Front Pharmacol. 2021 February 19;12:61 8411. https://pmc.ncbi.nlm.nih.gov/articles/PMC7933596/
Kim J De Jesus O Medication Routes of Administration . StatPearls August 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK568677/
Glagga A Pellegrini M Gupta Drug Absorption February 27, 2024. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK557405/
Herman T Santos C First-Pass Effect. November 3, 2023 StatPearls https://www.ncbi.nlm.nih.gov/books/NBK551679/
Drugs Undergoing Extensive First-Pass Metabolism. Clinical Pharmacology & Drug Therapy. KnowledgeDose December 7, 2019 https://www.knowledgedose.com/drugs-undergoing-extensive-first-pass-metabolism/#google_vignette
Chenmg L Wong H Food Effects on Oral Drug Absorption: Application of Physiologically-Based Pharmacokinetic Modeling as a Predictive Tool .Pharmaceutics. 2020 July 17;12(7): 672. https://pmc.ncbi.nlm.nih.gov/articles/PMC7408216/
Swarnalatha K Iswariya V Akash B Bhandari S Shirsha R Ramarao T (2024) A Comprehensive Review of Control Drug Release Delivery Systems: Current Status and Future Directions. International Journal of Pharmaceutical and Phytopharacological Research , 14( 2), 24-30. https://eijppr.com/article/a-comprehensive-review-of-controlled-drug-release-delivery-systems-current-status-and-future-direct-1hwbc1e8kyavpcj?html
Robinson J Gauger L Formulation of controlled-release products.J. Allergy Clin Immunol Volume 78 Number 4, Part 2 October 1986. https://www.jacionline.org/article/0091-6749(86)90045-X/pdf
Siepmann J Peppas N Modeling note drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Advanced Drug Delivery Reviews Volume 48, Issues 2-3, 11 June 2001, 139-157. https://www.sciencedirect.com/science/article/abs/pii/S0169409X01001120
Sielpmann J Peppas N Higuchi Equation: derivation, application, use and misuse. Int J Pharm. 2011 October 10;418(1): 6-12. Epub 2011 March 31. https://pubmed.ncbi.nlm.nih.gov/21458553/
Yang W-W Pierstorff E Reservoir-Base Polymer Drug Delivery Systems. SLAS Technology Volume 17, Issue 1, February 2012, 50-58. https://www.sciencedirect.com/science/article/pii/S2472630322016594
Yoshida R Sakai K Okano T Sakurai Y A New Model for Sero-Border Drug Release I. Hydrophobic Drug Release from Hydrophilic Polymeric Matrices. Polymer Journal, VOl. 23, No. 9, 1111-1121 (1991). https://www.nature.com/articles/pj1991133.pdf
Almoshari Y Osmotic Pump Drug Delivery Systems- A Comprehensive Review. Pharmaceuticals (Basel). November 18, 2022; 15(11): 1430. https://pmc.ncbi.nlm.nih.gov/articles/PMC9697821/
Sublingual administration. https://en.wikipedia.org/wiki/Sublingual_administration
Kim K Kerndt C Adnan G Schaller D Nitroglycerin. StatPearls July 31, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482382/
Buclin T Nicod M Kellenberger S Nitroglycerin pharmacokinetic parameters: Pharmacokinetics (Online content for students). https://sepia2.unil.ch/pharmacology/drugs/nitroglycerin/
Open RN Chapter 18 Administration of Parenteral Medications . Nursing Skills. National Library of Medicine https://www.ncbi.nlm.nih.gov/books/NBK593214/
Chapter 5: Intravenous Infusion in Applied By pharmaceutics & Pharmacokinetics, 6e https://accesspharmacy.mhmedical.com/content.aspx?bookid=513§ionid=41488023
Price G Patel D Drug Bioavailability July 30, 2023 StatPearls. National Library of Medicine https://www.ncbi.nlm.nih.gov/books/NBK557852/
Bourne D Pharmacokinetics and Biopharmaceutics Chapter 6: Intravenous Infusion https://www.boomer.org/c/p4/c06/c0602.php
IV fluids Risks/Benefits Cleveland Clinic https://my.clevelandclinic.org/health/treatments/21635-iv-fluids
Le J Drug Administration Merck Manual : Drug Administration November 2024 https://www.merckmanuals.com/home/drugs/administration-and-kinetics-of-drugs/drug-administration
Buxton I Chapter 2 Pharmacokinetics: The Dynamics of Drug Absorption, DIstribution, Metabolism, and Elimination In Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollman BC eds) McGraw Hill LLC (2023).
Intrathecal administration https://en.wikipedia.org/wiki/Intrathecal_administration
Blood-air barrier https://en.wikipedia.org/wiki/Blood%E2%80%93air_barrier
Labiris N Dolovich M Pulmonary drug delivery. Part 1: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J Clin Pharmacol. 2003 December; 56(610 ): 588-599. https://pmc.ncbi.nlm.nih.gov/articles/PMC1884307/
Guo Y Bera H Shi C Zhang L Cun D Yang M Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm Sin B. 2021 May 21;11(8): 2565-2584. https://pmc.ncbi.nlm.nih.gov/articles/PMC8424368/
Patton J Fishburn C Weers J The lungs as they Poor low Entry for Systemic Drug Delivery. Proceedings of the American Thoracic Society Volume 1, Issue 4 2004 https://www.atsjournals.org/doi/full/10.1513/pats.200409-049ta
King J Lowery D Physiology, Cardiac Output StatPearls National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK470455/
Herman T Santos C First-Pass Effect. StatPearls. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK551679/
Para-cellular transport. https://en.wikipedia.org/wiki/Paracellular_transport
Nickel S Clerkin C Selo M Ehrhardt C Transport mechanisms that the pulmonary mucosa: Implications for drug delivery. Expert Opinion on Drug Delivery Volume 13, Issue 5, 2016, 667-690. https://www.tandfonline.com/doi/full/10.1517/17425247.2016.1140144
Santiwarangkool S Akita H Khalil I Elwakil M Sato Y Kusumoto K Harashima H A study of the endocytosis mechanism and transendothelial activity of lung-targeted GALA-modified liposomes. Journal of Control Release Volume 307, August 10, 2019, 55-63. https://www.sciencedirect.com/science/article/abs/pii/S0168365919303207
Labiris N Dolovich Pulmonary drug delivery. Part II: The role of inhaler delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications Br J Clin Pharmacol. 2003 December;56(6): 600-612. https://pmc.ncbi.nlm.nih.gov/articles/PMC1884297/
Eixarch H Haltner-Ukomadu E Beisswenger C Bock U Drug Delivery to the Lung: Permeability and Physicochemical Characteristics of Drugs as the Basis for any Pulmonary Biopharmaceutical Classification System (pBCS) . Journal of Epithelial Biology & Pharmacology, 2010, 3, 1-14. https://benthamopenarchives.com/contents/pdf/JEBP/JEBP-3-1.pdf
Ibrahim M Verma R Garcia-Contreras L Inhalation drug delivery devices: technology update M.ed Devices (Auckl). 2015 February 12;8: 131-139. https://pmc.ncbi.nlm.nih.gov/articles/PMC4334339/
Borghardt J Kloft C Sharma A Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can Respir J. 2018 June 19;2018:2732017 https://pmc.ncbi.nlm.nih.gov/articles/PMC6029458/
Mucosa Cleveland Clinic https://my.clevelandclinic.org/health/body/23930-mucosa (Reviewed July 24, 2022).
Mucus Membrane https://en.wikipedia.org/wiki/Mucous_membrane
Song J Xu Z Xie L Shen J Recent Advances in Studying In In vitro Drug Permeation Across Mucosal Membranes. Pharmaceutics 17(2) 256, 2025. https://www.mdpi.com/1999-4923/17/2/256
Buccal in Sublingual Drug Delivery (Chapter 20) Pharmaceutics for Pharmacy Students. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3459§ionid=285256454
Wan g Z Chow M Capital review and appraisal of the current concepts and technologies for improvement of sublingual drug delivery. Ther Deliv 2014 July;5(7): 807-816. https://pubmed.ncbi.nlm.nih.gov/25287387/
BOC Sciences: Drug Delivery System for Buccal Administration. https://formulation.bocsci.com/resource/drug-delivery-system-for-buccal-administration.html
Turker S Onur E Ozer Y Nasal route in drug delivery systems. Pharm World Sci. 2004 June;26(3): 137-142. https://pubmed.ncbi.nlm.nih.gov/15230360/
Nasal administration. https://en.wikipedia.org/wiki/Nasal_administration
Chapman C Frey W Craft S Danielyan L Hallschmid M Schioth H Benedict C Intranasal Treatment central Nervous System Dysfunction in Humans. Pharm Res. 2012 November 8; 30(10): 2475-2044. https://pmc.ncbi.nlm.nih.gov/articles/PMC3761088/
Hua S Physiological and Pharmaceutical Considerations for Rectal Drug Formulations. Front Pharmacol. 2019 October 16;10: 1196. https://pmc.ncbi.nlm.nih.gov/articles/PMC6805701/
Rathi R Sanshita Kumar A Vishvakarma V Huanbutta K Singh I Sangnim T Advances in Rectal Drug Delivery Systems: Clinical Trials, and Patients Perspective. Pharmaceutics. 2022 October 17;14(10): 2210. https://pmc.ncbi.nlm.nih.gov/articles/PMC9609333/
Rectal and Vaginal Drug Delivery. Chapter 24. Pharmaceutics for Pharmacy Students. https://accesspharmacy.mhmedical.com/content.aspx?bookid=3459§ionid=285256969
Mofidfar M Abdi B Ahadian SS Mostafavi E Desai T Abbasi F Sun Y Manche E Ta C Flowers C Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int J Pharm 2021 July 26;607:120924. https://pmc.ncbi.nlm.nih.gov/articles/PMC8579814/
Bal-Ozturk A Ozcan-Bulbui E Gulekin H Cecen B Demir E Zarepour A Cetinel S Zarrabi A Application of Converging Science and Technology toward Ocular Disease Treatment. Pharmaceuticals 2023, 16(3), 445. https://www.mdpi.com/1424-8247/16/3/445
Santana C Matter B Patil M Silva-Cunha A Kompella U Corneal Permeability and Uptake of Twenty-Five Drugs: Species Comparison and Quantitative Structure-Permeability Relationships. Pharmaceutics. 2023 June 2;15(6): 1646. https://pmc.ncbi.nlm.nih.gov/articles/PMC10302615/
Moissev R Morrison P Steele F Khutroryanskiy V Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics. 2019 July 9;11(7): 321. https://pmc.ncbi.nlm.nih.gov/articles/PMC6681039/
Dosmar E Walsh J Doyel M Bussett K Oladipupo A Amer S Goebel K Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering (Basel). 2022 January 17;9(1): 41. https://pmc.ncbi.nlm.nih.gov/articles/PMC8772869/
Therapeutic Class Overview Ophthalmic Fluoroquinolones. 2014. https://www.medicaid.nv.gov/Downloads/provider/Ophthalmic Fluoroquin 2014 11.pdf
Compare Gentamicin versus Tobramycin. GoodRx. https://www.goodrx.com/compare/gentamicin-vs-tobrex
Chou T Hong B Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: background, effectiveness, tolerability, safety, and future applications. Ther Clin Risk Mnag. 2014 August 20: 10: 665-681. https://pubmed.ncbi.nlm.nih.gov/25187721/
Diaz-Tome V Bendicho-Lavilla Garcia-Otero X Varela-Fernandez R Martin-Pastor M Llovo-Taboada J Alsonso-Alonso P Aguiar P Gonzalez-Barica M Fernandex-Ferreiro A Oteo-Espinar F Antifungal Combination Wide Drops for Fungal Keratitis Treatment. Pharmaceutics. 2022 December 22;15(1): 35. https://pmc.ncbi.nlm.nih.gov/articles/PMC9866460/
Gung A Tran T Lim L Samarawickrama C Arnold J Gillies M Catt C Mitchell L Symons A ButteryR Cottee L Tumuluri K Beaumont P Local delivery of corticosteroids in clinical ophthalmology: A review. Clin Exp Ophthalmol. 2020 January 22; 48(3): 366-401. https://pmc.ncbi.nlm.nih.gov/articles/PMC7187156/
Furman B Prednisolone 2019, Reference Module in Biomedical Sciences. https://www.sciencedirect.com/topics/neuroscience/prednisolone-acetate
Weiser P (Medically Reviewed by Berger K on November 7, 2024) Loteprednol-Uses, Side Effects, and More. WebMD. https://www.webmd.com/drugs/2/drug-7319-835/lotemax-ophthalmic-eye/loteprednol-0-5-suspension-ophthalmic/details
Wingert A Liu S-H Lin J Sridhar J Non-Strobel anti-inflammatory agents for treating cystoid macular edema following cataract surgery. Cochrane Database Syst Rev. 2022 December 15;2022(12). https://pmc.ncbi.nlm.nih.gov/articles/PMC9754896/
Ghichloo I Gerriets Nonsteroidal Anti--Inflammatory Drugs (NSAIDs). StatPearls. May 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK547742/
Zhou L Zhan W Wei X Front Pharmacol. Clinical pharmacology andt pharmacogenetics of prostaglandin analogs and glaucoma. 2022 October 12;13. https://pmc.ncbi.nlm.nih.gov/articles/PMC9596770/
Lama P Reappraising the Safety of Beta-Blocker Therapy for Glaucoma. Glaucoma Today. May/June 2004 https://glaucomatoday.com/articles/2004-may-june/0504_01.html
Rob Schertzer Beta- Blockers for the Treatment of Glaucoma . West Coast Glaucoma. November 27, 2012. https://westcoastglaucoma.com/education/glaucoma/beta-blockers-for-the-treatment-of-glaucoma/
Akerman D Tropicamide vs. Cyclopentolate. Review of Myopia Management. March 1, 2022. https://reviewofmm.com/tropicamide-vs-cyclopentolate/
Farassat N Bohringer D Kuchlin S ...Lagreze W Low-dose Atropine for Myopia Control in Children (AIM): protocol for randomized, controlled double-blind multicentre, clinical trial with two parallel arms. BMJ Open. 2023 April 20;13(4). 87. https://pmc.ncbi.nlm.nih.gov/articles/PMC10124292/
Patton G Lee H Chemical Insights into Topical Agents in Intraocular Pressure Management: From Etiopathology to Therapeutic Approaches. Pharmaceutics. 2024 , 60(2), 274. https://www.mdpi.com/1999-4923/16/2/274
Aslam S Gapta V. Carbonic Anhydrase Inhibitors. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK557736/
Wang J Wang H Dang Rho-Kinase Inhibitors as Emergent Targets for Glaucoma Therapy .Ophthalmol Ther. 2023 October 14;12(6): 2943-2957. https://pmc.ncbi.nlm.nih.gov/articles/PMC10640453/
Chatzimichail E Christodoulaki E Konstas P Tsiropoulos G Amaxilati EE Gatzioufaz Z Panos G Roh Kinase Inhibitors in Glaucoma Management: Current Perspectives in Future Directions. Drug Design, Development and Therapy . April 2, 2025 volume 2025:19:2519-2531. https://www.dovepress.com/rho-kinase-inhibitors-in-glaucoma-management-current-perspectives-and--peer-reviewed-fulltext-article-DDDT
Rho kinase inhibitor https://en.wikipedia.org/wiki/Rho_kinase_inhibitor
Senst B Goyal A Basit H Borger J Drug Eluding Stent Compounds. StatPearls. July 4, 2023. https://www.ncbi.nlm.nih.gov/books/NBK537349/
Kwon H Park Local Delivery of Antiproliferative Agents via Stents. Polymers 2014, 6(3), 755-775. https://www.mdpi.com/2073-4360/6/3/755
Stone G Moses J Ellis S Schofer J Dawkins K Morice M-C Colombo A Leon M Safety and Efficacy of Serolimus-and Paclitaxel-Alluding Coronary Stents. N Engl J Med 2007;356 (10): 998-1008. https://www.nejm.org/doi/full/10.1056/NEJMoa067193
Moses J Leo M Popma J Fitzgerald P Holmes D O'Shaughnessy C Caputo R (SIRIUS Investigators) Serolimus-Eluding Stands versus Standard Stance in Patients with Stenosis in a Native Coronary Artery. N Engl J Med 2003;349(14): 1315-1323. https://www.nejm.org/doi/full/10.1056/NEJMoa035071
Serruys P Silber S Garg S van Geuns R Richardt G Buszman P Kelbaek H ...Windecker S Comparison of Zotarolimus-Eluting in everolimus-Eluting Coronary Stents. N Engl J Med 2010; 363(2). https://www.nejm.org/doi/full/10.1056/NEJMoa1004130
Stone G Rizvi A Newman W Mastali K Want J Caputo R Doostzadeh J for the SPIRIT IV Investigators. Serolimus-Eluting versus Paclitaxel-Eluting Stents in Coronary Artery Disease. N Engl J Med 2010;362(18): 1663-1674. https://www.nejm.org/doi/full/10.1056/NEJMoa0910496
Wu D Chen Q Chen ZX Han F Chen Z Wang Y The blood-brain barrier: Structure, regulation and drug delivery. Signal Transduction and Targeted Therapy 8, Article number: 217 (2023). https://www.nature.com/articles/s41392-023-01481-w
Enabling Novel Treatments for Nervous System Disorders by Improving Methods for Traversing the Blood Brain Barrier: Proceedings of the Workshop. Chapter 2 : Traversing the Blood-Brain Barrier: Challenges and Opportunities. 2008 https://www.ncbi.nlm.nih.gov/books/NBK507360/#_ncbi_dlg_citbx_NBK507360
Banks W From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 15, 275-292 (2016). https://www.nature.com/articles/nrd.2015.21
Dotiwala A McCausland C Samra N Anatomy, Head and Neck: Blood Brain Barrier. StatPearls. April 4, 2023 (Last Update). https://www.ncbi.nlm.nih.gov/books/NBK519556/
Cipolla M The Cerebral Circulation. Chapter 6 Barriers of the CNS. 2010 Morgan & Claypool Life Sciences. https://www.ncbi.nlm.nih.gov/books/NBK53084/
Kadry H Noorani B Cucullo L A Blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluid Barriers CNS 17, 69 (2020). https://fluidsbarrierscns.biomedcentral.com/articles/10.1186/s12987-020-00230-3
Luissint A-C Artus C Glacial F Ganeshmoorthy K Couraud P-O Tight junctions at the blood-brain barrier: physiological architecture in disease-associated dysregulation. Fluids and Areas of the CNS 9, Article number: 23 (2012). https://fluidsbarrierscns.biomedcentral.com/articles/10.1186/2045-8118-9-23
Sohet F Lin C Munji R Lee S Ruderisch N Soung A Arnold T Derugin N Vexler Z Yet F Daneman R LSR/Angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J Cell Biol (2015) 208 (6): 703-711. https://rupress.org/jcb/article/208/6/703/38116/LSR-angulin-1-is-a-tricellular-tight-junction
Xu L Nirwane A Yao Y Basement membrane and blood-brain barrier . Stroke Vasc Neurol. 2018 December 5;4(2): 78-82. https://pmc.ncbi.nlm.nih.gov/articles/PMC6613871/
Zhao Y Gan L Ren L Lin Y Ma C Lin X Factors influencing the blood-brain barrier permeability. Brain Research Volume 1788, August 1, 2022. https://www.sciencedirect.com/science/article/pii/S0006899322001615
Su W Pasternak G The Role Of Multidrug Resistance Associated Protein (MRP) in the Blood-Brain Barrier And Opioid Analgesia . Synapse. 2013 May 2;67(9): 609-619. https://pmc.ncbi.nlm.nih.gov/articles/PMC3752163/
Warner A Hathaway-Schrader J Lubker R Davies C Novice C Tetracyclines and bone: unclear actions with potentially lasting effects. Bone. 2022 March 3;159. https://pmc.ncbi.nlm.nih.gov/articles/PMC9035080/
Finerman G Milch R In Vito Binding of Tetracyclines to Calcium. Nature 198, 486-487 (1963). https://www.nature.com/articles/198486a0
Rosenthal A Fahey M Gohr C Burner T Konon I Daft L Mattson E Hirschmugl C Ryan L Simkin P Visibility avai Tetracycline Binding Method for Detecting Synovial Fluid Basic Calcium Phosphate Crystals. Arthritis Rheum. 2008 October;58(10): 3270-3274. https://pmc.ncbi.nlm.nih.gov/articles/PMC2574625/
Xu H Wang W Liu X Huang W Zhu C Xu Y Yang H Bai J Geng D Targeting strategies for bone diseases: signaling pathways and clinical studies. Signal Transduction and Targeted Therapy 8, Article number: 202 (2023). https://www.nature.com/articles/s41392-023-01467-8
Payne J Golub L Using Tetracyclines to Treat Osteoporotic/Osteopenic Bone Loss: From the Basic Science Laboratory to the Clinic. Pharmacol Res. 2010 October 16;63(II): 121-129. https://pmc.ncbi.nlm.nih.gov/articles/PMC3031719/
Radic M Belancic A Dogas H Vuckovic M Gelemanovic A Faour A Vlak I Radic Tetracyclines in Rheumatoid Arthritis: Dual Anti-Inflammatory and Immunomodulatory Roles, Effectiveness, and Safety Insights. Antibiotics 2025, 14(1), 65. https://www.mdpi.com/2079-6382/14/1/65
Shutter M Akhondi H Tetracycline. StatPearls. Last update: June 5, 2022. https://www.ncbi.nlm.nih.gov/books/NBK549905/
Busti A Herrington J The Mechanism for Tetracycline Associated Staining of the Teeth: Evidenced-Based Medicine Consult. Last Reviewed: October 2015. https://www.ebmconsult.com/articles/tetracycline-stains-teeth-mechanism
Sanchez A Rogers R Sheridan P Tetracycline another tetracycline-derivative staining of the teeth and oral cavity. Int J Dermatol. 2004 October;43(10): 709-715. https://pubmed.ncbi.nlm.nih.gov/15485524/
-
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development.
-
Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete.
-
Users should confirm the information contained herein with other sources.
-
This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site.
-
Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals.
-
Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration.
-
Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals.
-
-