|
Overview/Background
Master Gland--produces six major hormones, Stores two
hormones: Anatomy
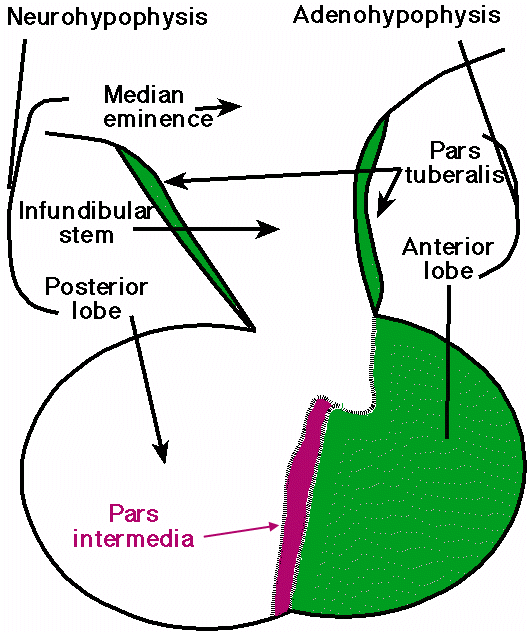
Midsagittal section of a human pituitary
gland; courtesy
of Robert H. Parsons, used with permission
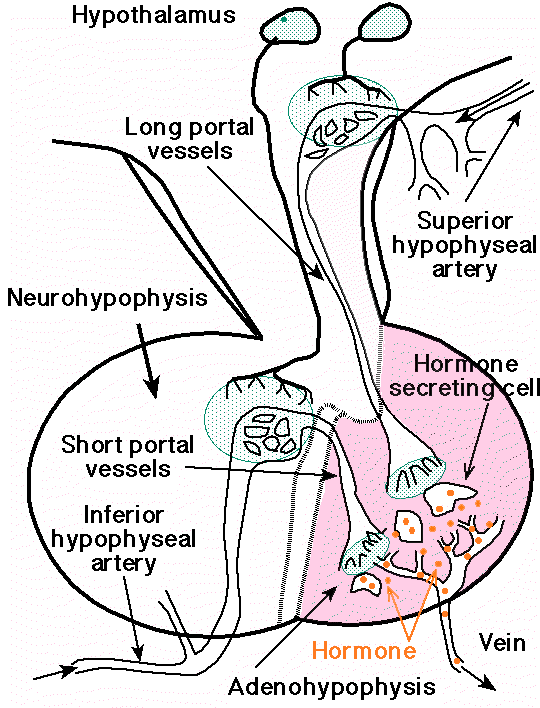
-
Sagittal section of a human pituitary,
showing the relationship of its blood supply to the hypothalamic
neurosecretory cells in the adenohypophysis. Neurosecretory
neurons are shown secreting releasing factors into the capillary
networks giving rise the long and short hypophyseal portal
vessels, respectively. The releasing hormones reach the
hormone-secreting cells of the anterior lobe via the portal
vessels.
-
courtesy of Robert H.
Parsons, Ph.D., Rensselaer Polytechnic Institute, used with
permission
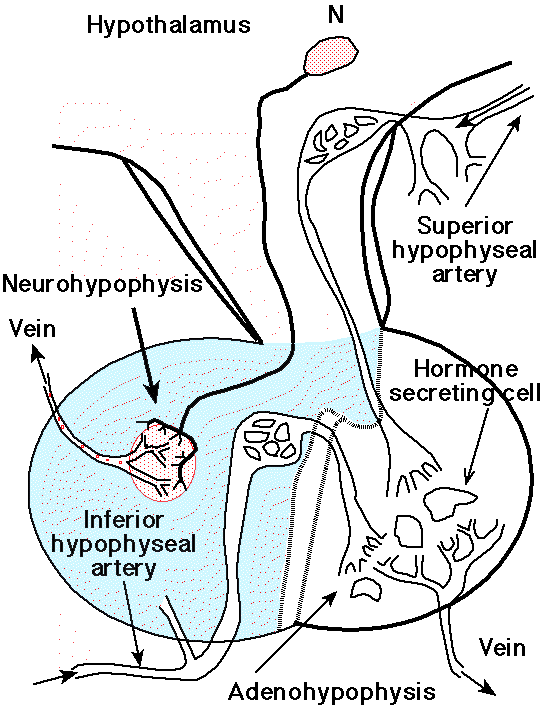
-
"Sagittal section of a human
pituitary, showing the relationship of its blood supply to the
neurosecretory cells of the supraoptic and paraventricular nuclei
of the hypothalamus. The neuron labeled N represent a
neurosecretory cell releasing ADH (antidiuretic hormone) or
oxytocin at its axon terminals into the capillaries giving rise to
the venous drainage of the posterior lobe. "
-
courtesy of Robert H. Parsons, used with
permission
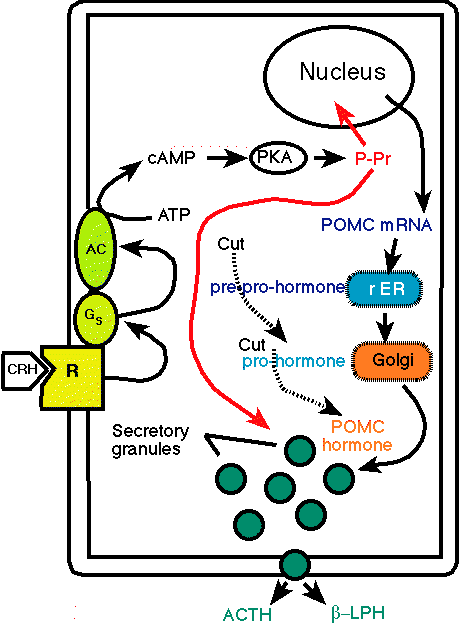
-
"Action of corticotrophin-releasing hormone (CRH)
on cells of the adrenal cortex. CRH binds to membrane receptors
(R), which are coupled to adenylate cyclase (AC) by stimulatory G
proteins (Gs). Adenylate cyclase is stimulated and cAMP rises in
the cell. cAMP activates protein kinase A (PKA), which then
phosphorylates proteins (P-Proteins) involved in stimulating ACTH
secretion and the expression of the POMC (proopiomelanocortin)
gene. The proteolytic processing of POMC occurs in the secretory
granulas where it is split into several hormones, ACTH (adrenocorticotrophic
hormone) and Beta-LPH (Beta-lipotropin). "
-
courtesy of Robert H. Parsons, Ph.D.,
Rensselaer Polytechnic Institute, used with permission
Pituitary and Hypothalamic Hormones
|
Pituitary Hormone
|
Hypophysiotropic
Hormone
|
|
Thyrotropin (TSH)
|
Thyrotropin-releasing
hormone (TRH) -- tripeptide
|
|
Adrenocorticotropin
(ACTH)
|
|
|
Luteinizing
hormone (LH)
|
Leutinizing
hormone-releasing hormone (LHRH) -- decapeptide
|
|
Follicle-stimulating
hormone (FSH)
|
LHRH --
decapeptide
|
|
Growth hormone (GH)
|
-
Growth hormone-releasing
hormone (GHRH) -- 44 amino acids
-
Growth hormone
release-inhibiting hormone (somatostatin,
GIH) -- 14 amino acids; somatostatin:
also inhibits TRH-stimulated TSH release
|
|
Prolactin
|
|
{adapted from Table
328-1: Biller, Beverly, M. K. and Daniels,
Gilbert, H. Neuroendocrine Regulation and Diseases of
the Anterior Pituitary and Hypothalamus, In
Harrison's Principles of
Internal Medicine 14th edition,
(Isselbacher, K.J., Braunwald, E., Wilson, J.D.,
Martin, J.B., Fauci, A.S. and Kasper, D.L., eds)
McGraw-Hill, Inc (Health Professions Division), 1998,
p. 197
Anatomy :
Pituitary
-
Pituitary gland
(hypophysis) resides within sella turcica of the sphenoid bone at the skull base
(weight = between 0.4 and 0.8 grams)
-
Midsagittal
section through human pituitary (above)
-
Sagittal
section of a human pituitary, showing the
relationship of its blood supply to the
hypothalamic neurosecretory cells in the
adenohypophysis (above)
-
Sagittal
section of a human pituitary, showing the
relationship of its blood supply to the
neurosecretory cells of the supraoptic and
paraventricular nuclei of the hypothalamus (above)
-
Pituitary gland
components:
-
Separated from brain by diaphragma
sella (dura mater extension) and by thin bone
layers from the sphenoid sinus anteriorly and
inferiorly
-
Sella lateral walls abut on the
cavernous sinuses (containing internal carotid
arteries & cranial nerves III, IV, V, and VI.
Recurrent
-
Optic chiasm located slightly
anterior to pituitary stalk -- just above
diaphragma sella.
Anatomy:
Hypothalamus
|
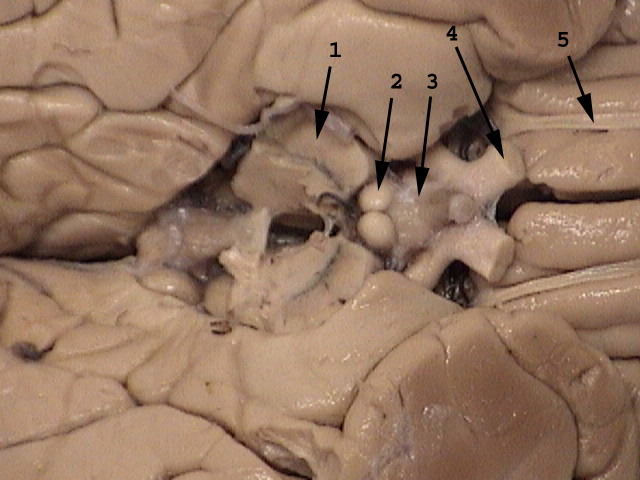
|
1. cerebral peduncle
2. mamillary body
3. floor of hypothalamus
4. optic nerve
5. olfactory tract
|
image source attribution:
University of Manitoba Anatomy
-
Hypothalamus:
-
anterior extension to
optic chiasm margin
-
posterior extension
including mammillary bodies
-
Separated from pituitary by:
-
Rounded inferior
hypothalamic base: tuber cinereum
-
Neuronal cell bodies of
supraoptic and periventricular
hypothalamic nuclei produce vasopressin
and oxytocin which are transported along
nerve axons (supraopticohypophyseal and
paraventriculohypophyseal tracts) to the posterior lobe
-
Hypothalamic-anterior
pituitary communication: chemical
-
Hypothalamic neuronal releasing
factors flow through the portal system to stimulate or inhibit
anterior pituitary hormone
production
-
Anterior
pituitary blood supply: (highest
blood flow of any tissue
--{0.8mL/g/min})
Mechanisms
of Hormone Action
-
GHRH,
Somatostatin, PRH, TSH, CRH, ACTH,
GnRH, FSH, LH, and Dopamine receptors:
-
GHRH,
CRH, GnRH, TSH, ACTH, FSH, LH, and Dopamine receptors
associated G protein-GTP
complex: adenylyl cyclase activation
: cAMP production: protein kinase activation:
intracellular protein phosphorylation:
hormonal effects
-
Dopamine
receptor -- Gi
protein coupling (lactotroph receptor system): decreased adenylyl cyclase activity:
decreased prolactin secretion
-
Growth
hormone: a-GTP
complex related to somatostatin receptors: potassium channels: inhibit growth hormone (GH) secretion
-
Thyrotropin-releasing
hormone: G protein complexes related to thyrotrophs' TRH receptors: phosphoinositide-specific phospholipase
C: cytoplasmic free calcium : stimulating TSH secretion
Growth hormone-releasing hormone
(GHRH, Sermorelin) & Growth hormone-releasing
peptides (GHRPs)
Somatostatin
(Growth hormone-inhibiting
hormone, Somatotropin release-inhibiting hormone)
-
Overview:somatostatin
-
14- and 28-amino acid peptide
forms, most widely distributed of the
hypothalamic releasing hormones
-
Localization:
hypothalamus & other CNS locations
-
periventricular & medial
pre-optic areas of anterior
hypothalamus
-
neurosecretory granules at nerve
terminals in the median eminence
-
serves as
neurotransmitter in the spinal
cord, cerebral cortex, brain stem
-- in addition to hormonal action
-
also
gastrointestinal & pancreatic
location
-
Inhibits growth hormone
release
-
Precursor: Prosomatostatin
-
Pharmacokinetics: somatostatin
-
Overview: octreotide:
-
Somatostatin analog -- longer
plasma elimination half-life (80 minutes)
-
> 40X more potent than
somatostatin in inhibiting growth hormone
release
-
only 2X more potent in
decreasing insulin secretion
-
Clinical
Use: octreotide
Following
subcutaneous doses every eight hours:
reduced symptoms from hormones secreted by
hormone-secreting tumors
|
acromegaly
|
carcinoid
syndrome
|
gastrinoma
|
|
glucagonoma
|
nesidioblastosis
|
watery
diarrhea
|
|
hypokalemia
|
achlorhydria
syndrome
|
"diabetic
diarrhea"
|
Growth Hormone (Somatotropin,
GH)
-
Overview: GH
-
Peptide
hormone: synthesized in anterior
pituitary
-
Growth
promotion:
-
Promotes lipolysis: adipose tissue
-
Promotes skeletal muscle growth
Growth
Hormone: Regulation
|
Type
of Agent
|
Stimulation
(+)
|
Inhibition
(-)
|
|
Hypothalamic
factors
|
GHRH
|
somatotropin
|
|
Biogenic amines
|
alpha-2 adrenergic receptor agonists
(clonidine, norepinephrine
|
beta- adrenergic agonists
|
|
|
beta-adrenergic receptor antagonists (e.g.,
propranolol)
|
alpha-2 adrenergic receptor antagonists
(e.g., yohimbine)
|
|
|
5-HT (serotonin)
stimuli (e.g.,L-tryptophan)
|
5-HT (serotonin)
receptor antagonists (e.g., cyproheptadine, methysergide)
|
|
|
Dopaminergic
stimuli (e.g., L-DOPA, apomorphine, bromocriptine)
|
Dopaminergic
antagonists (e.g., chlorpromazine)
|
|
Hormones
|
Decreased IGF-I
|
Increased IGF-I
|
|
|
Estrogen
|
Progestins
|
|
|
Vasopressin
|
Glucocorticoids
(acutely, glucocorticoids increase growth hormone
release)
|
|
|
Glucagon (cholinergic-mediated)
|
|
|
|
Hypoglycemia
(a-adrenergic mediated)
|
Increased
blood sugar
|
|
|
Decreased
free fatty acids
|
Increased
free fatty acids
|
|
|
Amino
acid (arginine; cholinergic-mediated)
|
|
|
Others
|
Exercise--a-adrenergic
mediated
|
Antimuscarinic
agents (e.g., atropine)
|
|
|
Stress--a-adrenergic
mediated
|
|
|
|
Sleep --cholinergic-mediated
|
|
|
|
Cholinergic-muscarinic
stimulation (e.g., pyridostigmine)
|
|
|
Adapted
from Table 328-3 Biller, Beverly, M. K. and
Daniels, Gilbert, H. Neuroendocrine Regulation
and Diseases of the Anterior Pituitary and
Hypothalamus, In Harrison's Principles
of Internal Medicine 14th
edition, (Isselbacher, K.J., Braunwald, E.,
Wilson, J.D., Martin, J.B., Fauci, A.S. and
Kasper, D.L., eds) McGraw-Hill, Inc (Health
Professions Division), 1998, p. 1979.
|
Thyrotropin-Releasing Hormone
(Protirelin, TRH)
Thyroid-Stimulating Hormone
(Thyrotropin, TSH)
Corticotropin-Releasing Hormone
(CRH)
Adrenocorticotropin
(corticotropin, ACTH, ACTH1-24 )
-
Overview:ACTH
-
peptide hormone;
-
synthesis site: anterior
pituitary
-
Major endocrine function:
stimulation of cortisol synthesis &
release from adrenal cortices
-
Synthetic corticotropin-derivative use clinically
to assess adrenocortical status
-
Chemistry:ACTH
-
single 39-amino acid
peptide
-
Synthetic, human ACTH1-24:
cosyntropin
-
Amino terminal sequence
(1-13): identical to
melanocyte-stimulating hormone (a-MSH)
-
Pharmacokinetics:ACTH
-
Porcine & synthetic
corticotropin: well absorbed following
intramuscular administration
-
Corticotropin: no oral
administration due to GI proteolysis
-
half-life: < 20 minutes
-
Tissue concentration: in
liver & kidney
-
Pharmacodynamics: ACTH
-
ACTH stimulates adrenal
cortex to produce glucocorticoid,
mineralocorticoid, & androgen.
-
ACTH increases cholesteryl
esters activity ( cholesterol:
pregnenolone
step: rate-limiting in steroid hormone
production)
-
ACTH promotes adrenal
hypertrophy & hyperplasia
-
corticotropin may cause
increased in skin pigmentation
-
Clinical
Use:ACTH
-
ACTH adrenal stimulation:
inadequate response in
adrenal-insufficiency
-
Cosyntropin may be used rule
out adrenal-insufficiency
-
Differentiation of
"late-onset" (non-classic)
congenital adrenal hyperplasia from
states of ovarian hyperandrogenism
-
21-hydroxylase deficiency: ACTH
stimulation:
incremental increase in
plasma 17-hydroxyprogesterone
(substrate for the deficient
enzyme)
-
11-hydroxylase deficiency: ACTH
stimulation: increase
11-deoxycortisol
-
3-b-hydroxy-D 5 steroid dehydrogenase
deficiency: ACTH stimulation: increase in
17-hydroxypregnenolone
-
Therapeutics:
corticotropin -- no advantage over direct
glucocorticoid administration
Gonadotropin-Releasing Hormone
(GnRH., luteinizing hormone-releasing hormone {LHRH};Gonadorelin)
Follicle-Stimulating Hormone (FSH)
-
Overview:FSH
-
Function:FSH
-
FSH + LH (luteinizing
hormone): gonadal function regulation--
mediated by increasing cAMP levels
in gonadal tissue
-
FSH --
principal function:
-
FSH site of action: immature
ovarian follicular cells: promoting
development of the mature follicle and
oocyte
-
Testes-- Site of action for
FSH: Sertoli cells, enhance
androgen-binding protein production
-
Modified
FSH molecules
-
Obtained from
postmenopausal women's urine
-
one agent --FSH-like
characteristics; 4% potency
-
another agent --LH-like
characteristics
-
FSH-LH combination: menotropins
-
Another preparation: also
from postmenopausal women's urine but
with no LH is urofollitropin
Leutinizing Hormone (LH)
-
Glycoprotein hormone (two chains)
-
Site of synthesis:
anterior pituitary
-
Major
physiological role:
-
regulation of gonadal
steroid hormone production
-
Site of action-- male:
-
Site of action -- female:
-
Note: no LH preparation
available for clinical use. Human
chorionic gonadotropin (very similar
structure) may be used as a leutinizing
hormone substitute
Gonadotropins (hMG, Menotropins
& FSH, Urofollitropin)
primary
amenorrhea
|
secondary
amenorrhea
|
polycystic
ovary syndrome
|
anovulatory
cycle
|
Hypothalamic/ Pituitary Agents
Generic
|
Trade
name
|
|
bromocriptine |
Parlodel |
|
chorionic
gonadotropin (hCG) |
generic, Profasi |
|
corticotropin |
generic, ACTH |
|
cosyntropin |
Cortrosyn |
|
desmopressin |
DDAVP, Stimate |
|
gonadorelin
acetate (GnRH) |
Lutrepulse |
|
gonadorelin
hydrochloride (GnRH) |
Factrel |
|
goserelin acetate |
Zoladex |
|
histrelin |
Supprelin |
|
leuprolide |
Lupron |
|
menotropins (hMG) |
Pergonal, Humegon |
|
nafarelin |
Synarel |
|
octreotide |
Sandostatin |
|
oxytocin |
generic, Pitocinit, Syntocinon |
|
pergolide |
Permax |
|
protirelin |
Thypinone,
Relefact TRH |
|
sermorelin (GHRH) |
Geref |
|
somatrem |
Protropin |
|
somatropin |
Humatrope,
Nutropin |
|
thyrotropin (TSH) |
Thytropar |
Human Chorionic Gonadotropin
(hCG)
-
Overview:hCG
-
Pharmacokinetics:hCG
-
Pharmacodynamics:hCG
-
Clinical
Uses:hCG
-
Diagnostic:hCG
-
pre-pubertal
boys with undescended gonads: hCG can distinguish
between retained testes
(cryptorchid) and retracted
testes (pseudocryptorchid)
-
if transient
testicular descent occurs
with hCG administration: permanent
pubertal descent
-
if transient
testicular descent does
not occur with hCG
administration,
orchiopexy will be
required to insurer
spermatogenesis
-
Constitutional puberty
delay vs. hypogonadotropic
hypogonadism: distinguished using
repetitive hCG administration
-
Therapeutic:hCG
-
hCG + human menotropin: ovulation in women
with hypogonadotropic
hypogonadism or as part of in
vitro fertilization
approach
-
hCG: testicular
testosterone stimulation in men
with hypogonadotropic
hypogonadism (increased
intratesticular testosterone:
promotes
spermatogenesis; menotropins
often also required for
fertility)
-
Toxicity:hCG
-
headache, edema,
gynecomastia, pretentious puberty,
depression, hCG antibody production
(rare)
-
Contraindications:hCG
Prolactin
Bromocriptine and Other Dopamine
Agonists
-
Acromegaly:
-
bromocriptine +/-
pituitary surgery, radiation
therapy, octreotide: treatment of
acromegaly
-
bromocriptine
responsiveness in these patients
depends on prolactin as well
as growth hormone secretion
by pituitary tumor
-
Parkinson's Disease:
Posterior Pituitary Hormones
|