-
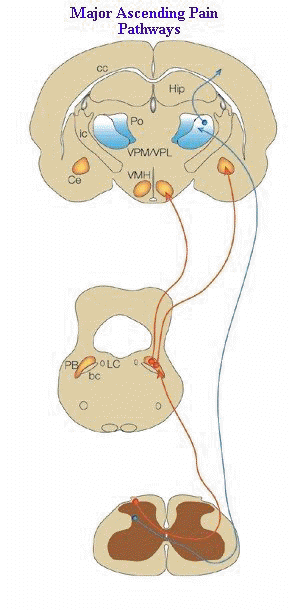
"There are two primary ascending nociceptive pathways.
-
These
are the spinoparabrachial pathway (red), which originates from the superficial
dorsal horn and feeds areas of the brain that are concerned with affect, and
the spinothalamic pathway (blue), which probably distributes nociceptive information
to areas of the cortex that are concerned with both discrimination and affect.
-
Many more less prominent pathways could be added2, 5, 6, 68-72.
-
(A,
adrenergic nucleus; bc, brachium conjunctivum; cc, corpus
callosum; Ce, central nucleus of the amygdala; Hip,
hippocampus; ic, internal capsule; LC, locus coeruleus; PB,
parabrachial area; Po, posterior group of thalamic nuclei; Py,
pyramidal tract; RVM, rostroventral medulla; V, ventricle; VMH,
ventral medial nucleus of the hypothalamus; VPL, ventral
posteriolateral nucleus of the thalamus; VPM; ventral
posteriomedial nucleus of the thalamus.)"
-
Figure adapted from: Nature Reviews Neuroscience 2;
83-91
(2001): THE MOLECULAR DYNAMICS OF PAIN CONTROL Nature © Macmillan Publishers Ltd 2001 Registered No. 785998 England
|
 |
-
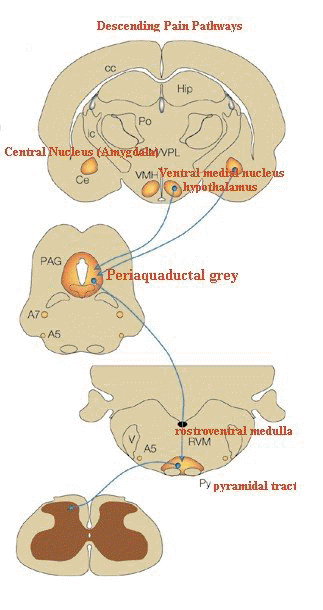
"The descending pathway highlighted
originates from the amygdala and hypothalamus and terminates
in the periaqueductal grey (PAG).
-
Neurons project from here to
the lower brainstem and control many of the antinociceptive
and autonomic responses that follow noxious stimulation.
-
(A,
adrenergic nucleus; bc, brachium conjunctivum; cc, corpus
callosum; Ce, central nucleus of the amygdala; Hip,
hippocampus; ic, internal capsule; LC, locus coeruleus; PB,
parabrachial area; Po, posterior group of thalamic nuclei; Py,
pyramidal tract; RVM, rostroventral medulla; V, ventricle; VMH,
ventral medial nucleus of the hypothalamus; VPL, ventral
posteriolateral nucleus of the thalamus; VPM; ventral
posteriomedial nucleus of the thalamus.)"
-
Figure adapted from: Nature Reviews Neuroscience 2;
83-91
(2001): THE MOLECULAR DYNAMICS OF PAIN CONTROL Nature © Macmillan Publishers Ltd 2001 Registered No. 785998 England
|
 |
|
Membrane Bilayer
Structure
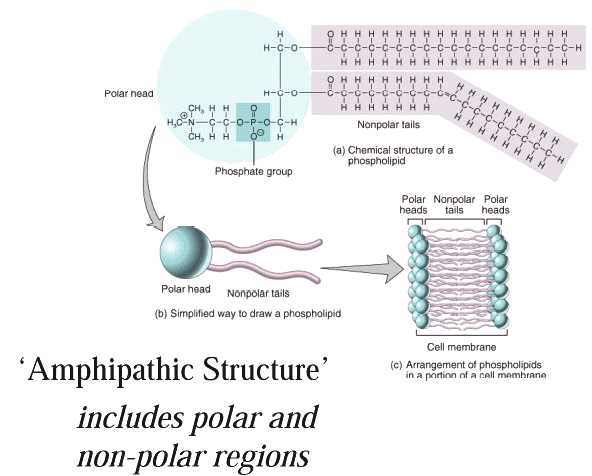
Representation of the lipophilic,
hydrophobic core characteristic of biomembrane
structure.
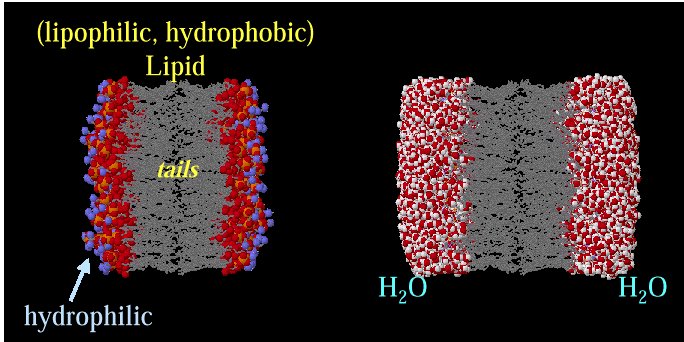
Above images courtesy of Professor Steve Wright and the University
of Arizona (c) 2001, used with permission.
|
|