|
|
|
|
-
Microbiome and Variation in Drug Bioavailability -
 Following
oral administration of the drug, multiple "barriers"
may limit ready drug access to its site of action.15
Following
oral administration of the drug, multiple "barriers"
may limit ready drug access to its site of action.15
-
Some of these barriers include limits to drug absorption both in the stomach and small intestine.
-
Intestinal and liver cytochrome P450 drug metabolizing systems may also expose the drug to enzymes that transform an active drug to a less active or inactive form.
-
One mechanism by which a drug gets to the cell but ultimately shows reduced activity involves "multidrug-efflux transporter Pgp."
-
This system may pump a drug out of the cell and limit its activity.
-
-
As noted earlier, intestinal bacteria also can modify drugs directly and also by changing the individual's metabolism. Some of these interactions may extend to parenterally administered drugs as well.15
-
-
Drug bioavailability, a clinically central objective, is affected substantially both by interactions between the drug and the cytochrome P450 microsomal metabolizing system as well as by the drug-efflux transporter.
-
 Bioavailability variability may be
substantially responsible for both "therapeutic
failure" and "toxicity." 15
Bioavailability variability may be
substantially responsible for both "therapeutic
failure" and "toxicity." 15
-
This conclusion is not surprising given that if one considers all drugs on the market, cytochrome P450 enzymes (isoforms) metabolized about 75% of these agents.
-
About half of the time, a particular cytochrome P450 family, CYP3A, is a principal contributor.15
-
The importance of CYP3A itself or more accurately a CYP3A isoform (e.g. CYP3A4) may be inhibited or activated and thus contribute to either an elevation or reduction in expected drug levels.
-
-
-
One approach to assess the role of the gut microbiome and drug toxicity involves the utilization of "germ-free" (GF) mice.
-
An example utilizes the immunosuppressent agent mycophenylate mofetil (MMF).
-
This agent is effective but its administration is associated with noteworthy side effects (toxicities).19
-
Treatment of mice with MMF resulted both in notable weight loss due to reduced body fat and muscle and colonic inflammation.20
-
Furthermore, MMF administration changed the gut microbiome composition in the direction of reduced overall diversity, with increased Proteobacteria (particularly Escherichia/Shigella) as well as "enrichment" of genes associated with lipopolysaccharide (LPS) biosynthesis.20
-
-
 Reduction
in side effects however was noted when MMF was
administered to GF mice, which suggested that
the gut microbiome might be important in
emergent adverse effect.19
Reduction
in side effects however was noted when MMF was
administered to GF mice, which suggested that
the gut microbiome might be important in
emergent adverse effect.19
-
Administration of broad-spectrum antibiotics prevented as well as reversed MMF-associated colonic inflammation and weight loss.20
-
-
-
Another example in which the gut microbiome may modify a drug involves the enzyme tyrosine decarboxylase and inactivation of the Parkinson's disease drug levodopa (L-DOPA).
-
Tyrosine decarboxylase is expressed by Enterococcus and Lactobacillus.
-
 Extended treatment with L-DOPA appears to
increase tyrosine decarboxylase gene expression
consistent with reduced L-DOPA treatment
effectiveness over time.19
Extended treatment with L-DOPA appears to
increase tyrosine decarboxylase gene expression
consistent with reduced L-DOPA treatment
effectiveness over time.19
-
-
Yet another possible interaction involves E. coli expression of tyrosine oxidase which may bind amphetamine thus possibly modifying drug action.19
-
-
Xenobiotics (ingested foreign substances e.g. drugs) and the Gut Microbiome19 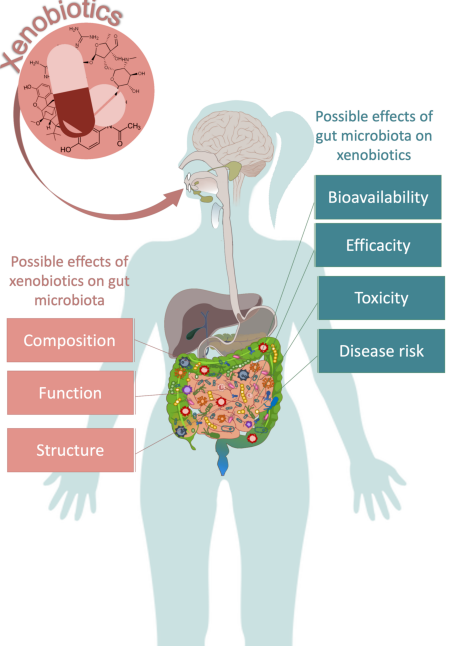
-
Attribution: Figure 2 in:
-
Leprun PMB Clarke G The gut microbiome and pharmacology: a prescription for the therapeutic targeting of the gut-brain axis. Current Opinion in Pharmacology (December 2019) 49:17-23 https://www.sciencedirect.com/science/article/abs/pii/S1471489218301516
-
-
-
"How the Gut Microbiota Interacts with Medications" -
Barron M How the Gut Microbiota Interacts with Medications (March 17, 2023)
-
American Society for Microbiology Youtube Channel (https://www.youtube.com/@asmicrobiology)
-
-
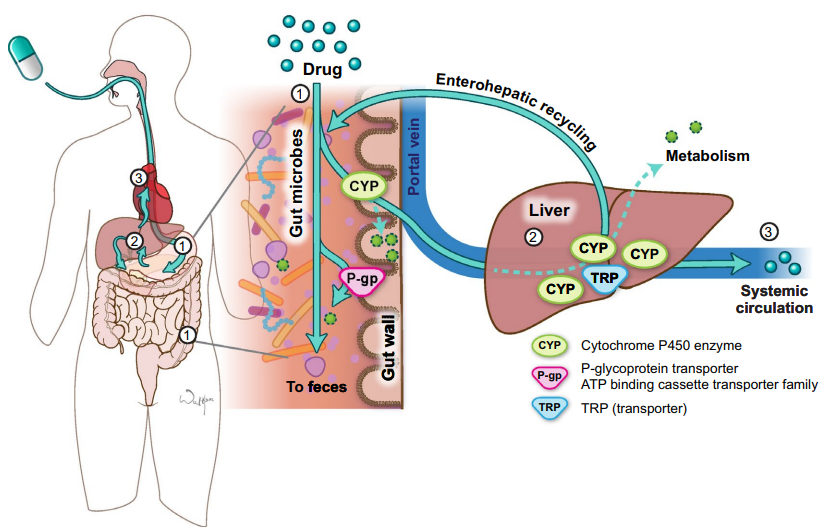
-
"Pathway of an orally administered drug.18
-
When a drug is administered orally (1), it encounters gut microbes, cytochrome P450 (CYP) enzymes and transporters (TRP) such as P-glycoprotein (P-gp) in the small and large intestine.
-
Some drug will be lost in the feces in these processes.
-
The drug that survives the small intestine will then travel through the portal vein to the liver where it encounters more CYPs and TRP and more drug may be lost to metabolism (2).
-
Some drugs undergo enterohepatic recycling in which drug conjugates are transported from the liver back to the intestine where they encounter microbes, CYP, and TRP again.
-
 The
amount of drug that enters
the systemic circulation is
a fraction of what was
originally ingested (3). ATP
adenosine triphosphate."18
The
amount of drug that enters
the systemic circulation is
a fraction of what was
originally ingested (3). ATP
adenosine triphosphate."18
-
-
-
-
References
Maher TJ Kiel D Chapter 6 G-Protein-Coupled Receptors in Foye's Principles of Medicinal Chemistry (Roche VF Zito SW Lemke TL Williams DA, eds) 8e Wolters Kluwer 2020.
Alenghat FJ Golan DE Chapter 1 Drug-Receptor Interactions in Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy (Golan DE Armstrons EJ Armstong AW, eds) 4e Wolters Kluwer 2017.
von Zastrow M Chapter 2 Drug Receptors & Pharmacodynamics in Basic & Clinical Pharmacology ( Katzung BG Vanderah TW, eds) 14e McGraw Hill 2021.
Flood P Shafer SL Chapter 2 Basic principles of Pharmacology in Stoelting's Pharmacology & Physiology Anesthetic Practice (Flood P Rathmell JP Urman RD, eds) 6e 2022.
Manning DR Blumenthal DK Chapter 3 Pharmacodynamics: Molecular Mechanisms of Drug Action in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollmann BC, eds) 14e McGraw-Hill 2023.
Burchum JR Rosenthal LD Charles C Chapter 5 Pharmacodynamics Lehne's Pharmacology for Nursing Care 11e Elsevier 2022.
Katzung, BG Introduction: Chapter 1 The Nature of Drugs & Drug Development & Regulation in Basic and Clinical Pharmacology (Katzung BG Vanderah TW, eds) 15e McGraw Hill 2021.
Geroge Jr AL Neilson EG Chapter 309 Cell Biology and Physiology of the Kidney in Harrison's Principles of Internal Medicine (Loscalzo J Kasper DL Longo DL Fauci AS Hauser SLs Jameson JL, eds) 21e 2022.
Burchum JR Rosenthal LD Charles C Chapter 4 Pharmacokinetics Lehne's Pharmacology for Nursing Care 11e Elsevier 2022.
Singer SJ Nicolson GL The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972 Feb 18; 175(4023): 720-731.
Watson H Biological membranes Essays Biochem (2015) 59, 43-70. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626904/pdf/bse0590043.pdf
Holford NHG Chapter 3 Pharmacokinetics & Pharmacodynamics: Rational Dosing & the Time Course of Drug Action in Basic and Clinical Pharmacology (Katzung BG Vanderah TW, eds) 15e McGraw Hill 2021.
Buxton ILO Chapter 2 Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollmann BC, eds) 14e McGraw-Hill 2023.
Baca QJ Golan DE Chapter 3 Pharmacokinetics in Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy (Golan DE Armstrons EJ Armstong AW, eds) 4e Wolters Kluwer 2017.
Tsunoda SM Dorrestein PC Knight Rob Chapter 6 The Gastrointestinal Microbiome and Drug Response in Goodman & Gilman's The Pharmacological Basis of Therapeutics (Brunton LL Knollmann BC, eds) 14e McGraw-Hill 2023.
The Editors Chapter 1 The Practice of Medicine in in Harrison's Principles of Internal Medicine (Loscalzo J Kasper DL Longo DL Fauci AS Hauser SLs Jameson JL, eds) 21e 2022.
Lam KN Slexander M Turnbaugh PJ Precision Medicine Goes Microscoptic: Engineering the Microbiome to Improve Drug Outcomes. Cell Host & Microbe July 10, 2019. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6709864/
Tsunoda SM Gonzales C Jarmujsch AK Momper JD Ma JD Contribution of the Gut Microbiome to Drug Disposition, Pharmcokinetic and Pharmacodynamic Variability. Clinical Pharmacokinetics (2021) 60: 971-984. https://link.springer.com/article/10.1007/s40262-021-01032-y
Leprun PMB Clarke G The gut microbiome and pharmacology: a prescription for the therapeutic targeting of the gut-brain axis. Current Opinion in Pharmacology (December 2019) 49:17-23 https://www.sciencedirect.com/science/article/abs/pii/S1471489218301516
Flannigan KL Taylor MR Pereira SK Rodriguiez-Arguello J Moffat AW Alston L Wang XM Poon KK Beck PL Rioux KP Jonnalagadda M Chelikani PK Galipeau HJ Lewis IA Workentine ML Greenway SC Hirota SA An intact microbiota is required for the gastrointestinal toxicity of the immunosuppressant mycophenolate mofetil J Heart Lung Transpl (2018) 37(9): 1047-1059. https://www.sciencedirect.com/science/article/abs/pii/S105324981831475X
|