|
|
|
|
-
-
Gastrointestinal Microbiome and Pharmacology -
Gastrointestinal microbiome consists of bacteria, viruses, fungi and microbial eukaryotes.15
-
Greater than 90% of the gut microbiota are described by members of two bacteria phyla, Bacteroidetes and Firmicutes.
-
Bacterioides biacutis 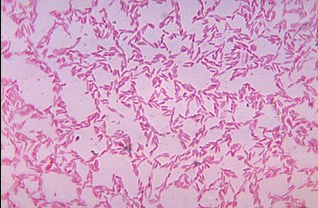
-
"Bacterioides biacutis-one of many commensal anaerobic Bacteroides species in the gastrointestinal tract-cultured and blood agar medium for 48 hours.
-
"The phylum Bacteroidetes consists of 3 large classes of Gram-negative, nonsporeforming, anaerobic or aerobic and rod-shaped bacteria."15
-
Image obtained from the CDC Public Health Image Library (https://phil.cdc.gov/default.aspx)
-
Attribution: US gov, Public Domain via Wikimedia Commons
-
https://commons.wikimedia.org/wiki/File:Bacteroides_biacutis_01.jpg
-
-
Bacillus subtilis (Firmicutes/Bacillota) 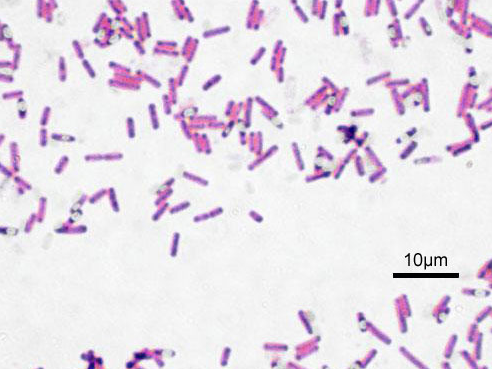
-
"Microscopic image of Bacillus subtilis. Gram staining, magnification: 1000. The oval unstained structures or spores.
-
Attribution: Y tambe (original uploader), CC BY-SA 3.0 <http://creativecommons.org/licenses/by-sa/3.0/>, via Wikimedia Commons
-
https://commons.wikimedia.org/wiki/File:Bacillus_subtilis_Gram.jpg
-
-
-
The gut microbiomes is described by over 1000 distinct species with substantial differences between individuals.15
-
 The challenge that physicians face in diagnostics and
therapeutics is driven by the recognition that phenotypes are
not defined solely on the basis of genes alone but rather on
interactions between genes and gene products and further by the
interactions between genetics and environmental factors.16
The challenge that physicians face in diagnostics and
therapeutics is driven by the recognition that phenotypes are
not defined solely on the basis of genes alone but rather on
interactions between genes and gene products and further by the
interactions between genetics and environmental factors.16
-
For example, epigenetic influences gene expression despite cells having the same DNA sequences.
-
Epigenetic-induced changes appear associated with a number of disease processes.16
-
-
Proteomics describes relationships between large numbers of cellular proteins and disease.
-
These interactions allow enhancement of cellular biological potentialities beyond the 23,000 human genes in the genome by means of posttranslational processing, alternate splicing, and posttranslational modifications map to many unique and therapeutically relevant consequences.16
Microbiomics emphasize the relationship between resident human microbes and their relationship to human health.
-
The significance of such interactions is suggested by the number of genes in the human haploid genome (about 23,000) compared to the 3-4 million genes associated with resident microbes in humans.
-
-
Maturation of the immune system, along with metabolic balance, disease susceptibility and brain function appear to be influenced by those microbes associated with skin surfaces and human mucosal surfaces.16
-
Pharmacomicrobiomics Venn Diagram Microbiota
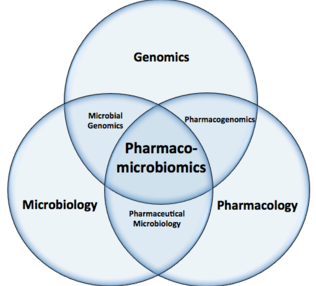
-
Attribution: Arjunsbaghela, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
-
"This Venn diagram shows that pharmacomicrobiomics is a sub-field of genomics, microbiology and pharmacology."
-
https://commons.wikimedia.org/wiki/File:Pharmacomicrobiomics_Venn_Diagram.png
-
Microbiota and their "Theatre of Activity"
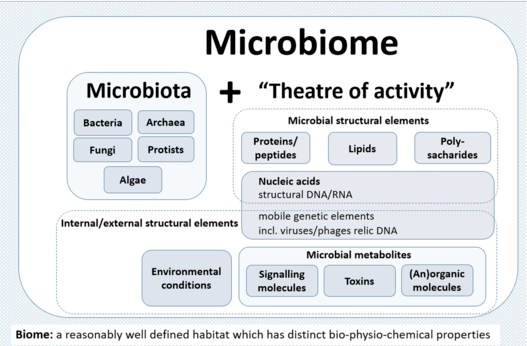
-
Attribution: Berg G Rybakova and 33 others Composition of the term microbiome containing both the microbiota (community of microorganisms and their 'theatre of activity' (structural elements, metabolites/signal molecules, and the surrounding environmental conditions)"
-
Attribution: Gabriele Berg, Daria Rybakova and 33 others, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
-
Berg G. Rybakova D Fischer D. et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103 (2020).
-
https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-020-00875-0
-
-
"Human Gut Microbes Metabolize Xenobiotics" 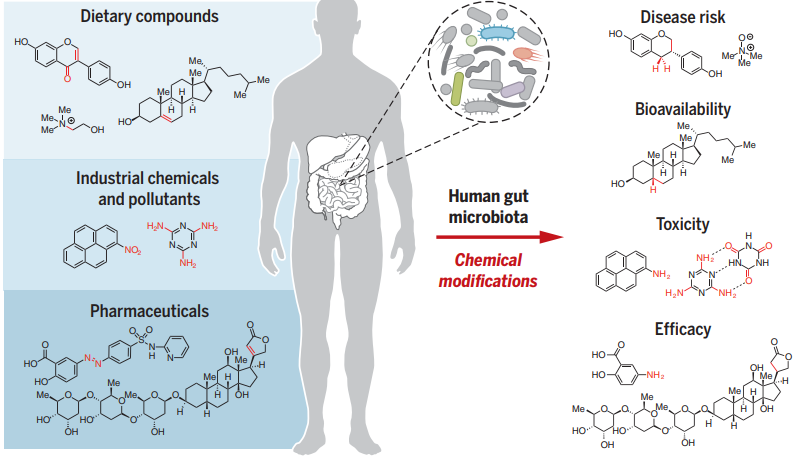
-
"The microorganisms that inhabit the human gut alter the chemical structures of ingested compounds, including dietary components, industrial chemicals, and drugs.
-
 "These
changes affect xenobiotic toxicity, biological
activity, and bioavailability.
"These
changes affect xenobiotic toxicity, biological
activity, and bioavailability. -
"The gut microbial enzymes responsible for many of these transformations are poorly understood. Me, methyl."
-
Attribution: Koppel N, et al. Chemical transformation of xenobiotics by the human gut microbiota. Science, 2017, 356 (6344): eaag2770. https://www.science.org/doi/10.1126/science.aag2770.
-
-
-
The Microbiome and Disease -
The microbiome seems to be involved in a number of human diseases, although the underlying mechanism remains to be elucidated.15
-
 Diseases
in which microbiome have been implicated
includes:
Diseases
in which microbiome have been implicated
includes:
-
Autoimmune diseases
-
Autism
-
Hepatic disease
-
Cancer
-
Cardiovascular Disease.
-
-
About 16% of all cancers in the United States appear closely related to elements of the microbiome. 15
-
Microbiome elements likely associated with cancer.:
-
hepatitis C
-
Heliobacter pylori
-
Human papilloma virus.
-
-
-
DNA cleavage and potentially associated colorectal cancer may result from colibactin-producing Escherichia coli.15
-
Colibactin is considered a secondary metabolite associated with certain bacterial strains present in the human gut.17
-
Colibactin-producing bacteria appear correlated with human colorectal cancer.
-
Colibactin exhibits a heterodimeric structure with two DNA-reactive residues allowing formation of interstrand adenine cross-links (alkylation) between opposing DNA strands.17
-
Colibactin + E. coli → Colibactin-DNA Cross-link17 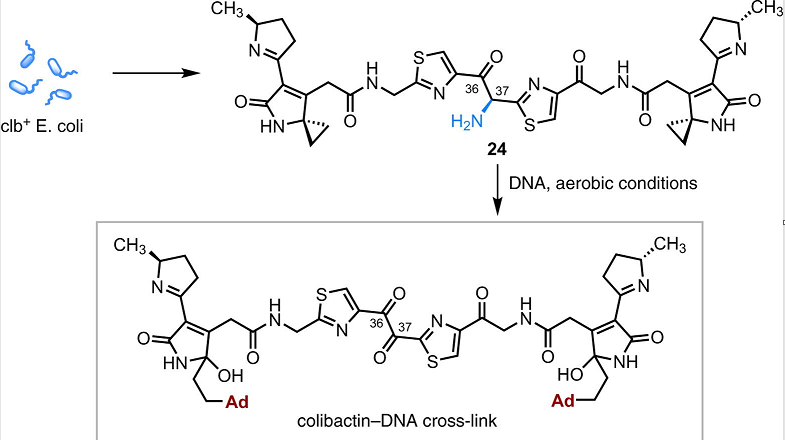
-
Attribution: Wernke KM Xue M Tirla A Kim CS Crawford JM Herzon SB Structure and Bioactivity of Colibactin Bioorganic & Medicinal Chemistry Letters 30(15) 1 August 2020.
-
https://www.sciencedirect.com/science/article/abs/pii/S0960894X20303905 ;
-
-
-
-
-
Interactions Between the Microbiome and Drugs -
Drugs and their Interactions with the Bodies' Microbiome
-
 Variation
in an individual's response to a drug is
affected by the gut microbiome.
Variation
in an individual's response to a drug is
affected by the gut microbiome. -
The microbiome can influence almost every aspect of a drugs interaction in the body.
-
For example, the microbiome can affect:
-
Metabolism of Xenobiotics
-
Influence drug metabolism by modulating phase I (P450 drug metabolizing system) enzymes
-
and phase II (e.g. conjugation reactions)
-
-
Influencing drug transporters
-
Affecting enterohepatic circulation Involvement in drug-pharmacodynamic interactions.15,17,18
-
-
-
Gut bacteria can participate in direct drug metabolism.
-
For example, digitoxin, a cardiac glycoside, can be inactivated by the microbe Eggerthella lenta and such inactivation could be important given that variability in drug concentration is especially important if the drug exhibits a narrow therapeutic index.
-
Another example of a narrow therapeutic range agent, tacrolimus appears linked to Faecalibacterium prausnitzii in that patients (kidney transplant) requiring higher tacrolimus doses also exhibited elevated amounts of Faecalibacterium prausnitzii.15,18
-
Tacrolimus (left); Digitoxin (right)
-
Digitoxin
-
Attribution: MarinaVladivostok, CC0, via Wikimedia Commons
-
https://commons.wikimedia.org/wiki/File:Digon_ball-and-stick.png
-
Tacrolimus
-
Attribution: Brenton, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
-
https://commons.wikimedia.org/wiki/File:Tacrolimus-1YAT-ball-and-stick-model.png
-
-
-
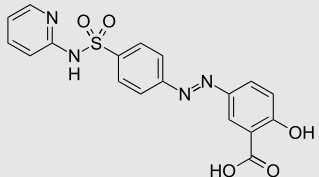
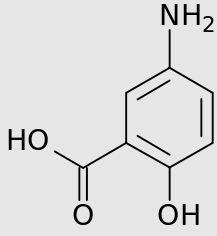
An example in which microbiome gut bacteria enzymic activity is required for drug activation is illustrated by the prodrug sulfasalazine.
-
Prodrugs are defined as drugs that in their ingested form are not biologically active but upon metabolic transformation a pharmacologically active form results.
-
In this instance the azo bond of sulfasalazine is reduced by gut bacteria yielding the active form, 5-aminosalicylate (mesalamine).
-
Sulfasalazine (left); Mesalamine (5-aminosalicylate; right)
-
Sulfasalazine
-
Attribution: Unknown sourceUnknown source, Public domain, via Wikimedia Commons
-
Mesalamine
-
Attribution: User:Mysid, Public domain, via Wikimedia Commons
-
-
-
-
-
References
|
|