|
|
|
|
|
|
|
-
Foundations of Drug Metabolism
-
Type of Conjugation
Endogenous Reactant
Transferase (Location)
Types of Substrates
Examples
Glucuronidation
UDP glucuronic acid
UDP glucuronosyl transferase (microsomal)
phenols, alcohols, carboxylic acids, hydroxylamines, sulfonamides
morphine, acetaminophen, diazepam, digitoxin, digoxin, meprobamate
Acetylation
Acetyl-CoA
N-Acetyl transferase (cytosol)
Amines
Sulfonamides, isoniazid, clonazepam, dapsone, mescaline
Glutathione conjugation
Glutathione
GSH-S-transferase (cytosolic, microsomes)
Epoxides, nitro groups, hydroxylamines
Ethycrinic acid, bromobenzene
Sulfate conjugation
Phosphoadenosyl phosphosulfate
Sulfotransferase (cytosol)
Phenols, alcohols, aromatic amines
Estrone, 3-hydroxy coumarin, acetaminophen, methyldopa
Methylation
S-Adenosyl-methionine
Transmethylases (cytosol)
Catecholamines, phenols, amines, histamine
Dopamine, epinephrine, histamine, thiouracil, pyridine
-
Adapted from Table 4-3, Correia, M.A., Drug Biotransformation. in Basic and Clinical Pharmacology, (Vanderah T, ed) Appleton-Lange, 16e 2023
-
-
Phase II Reactions
-
Phase II reactions involve coupling the parent drug (or its Phase I metabolite) with an endogenous polar molecule, effectively polarizing the compound for excretion.
-
Major Phase II pathways include glucuronidation, sulfation, acetylation, methylation, and glutathione or amino acid conjugation.4
-
-
These reactions are catalyzed by specific transferase enzymes, usually located in the cytosol of liver cells (with the exception that glucuronidation enzymes are mainly in the endoplasmic reticulum).
-
Phase II conjugates are typically highly water-soluble and pharmacologically inactive, so Phase II is generally considered a detoxification/inactivation step.5
-
There are occasional exceptions where a Phase II metabolite has activity.
-
Morphine-6-glucuronide is an active metabolite of morphine
-
Usually activity is greatly diminished or abolished after conjugation.
-
-
-
Because Phase II increases hydrophilicity substantially, these conjugated metabolites are readily excreted in urine (if small) or bile (if larger molecular weight).
-
If a drug molecule already possesses a suitable functional group, it may directly enter a Phase II pathway without needing Phase I.
-
If Phase II yields a metabolite that still has lipophilicity, it might undergo further Phase I or Phase III processing. Phase I and II are thus not strictly sequential; they can occur in parallel or in reverse order for some substances.4
-
-
-
-
-
-
 Glucuronidation
is the most common phase II pathway and is catalyzed by
UDP-glucuronosyltransferases (UGTs).
Glucuronidation
is the most common phase II pathway and is catalyzed by
UDP-glucuronosyltransferases (UGTs).-
Example glucuronidation reaction
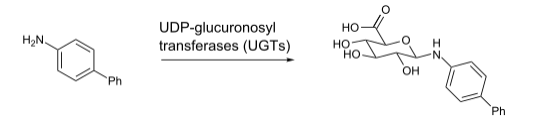
-
Example of N-glucuronidation of 4-4-aminobiphenyl by UGT1A4.
-
Attribution
-
Glucuronidation. https://en.wikipedia.org/wiki/Glucuronidation
-
-
-
-
Glucuronidation involves the transfer of glucuronic acid (from UDP-glucuronic acid) to substrates with suitable functional groups (–OH, –COOH, –NH2, –SH).
-
Many drugs and endogenous substances undergo glucuronidation, including bilirubin, acetaminophen, morphine, lorazepam, nonsteroidal anti-inflammatory drugs and others.6
-
The resulting glucuronide conjugates are usually inactive and readily excreted.
-
-
-
A clinically important aspect is the developmental expression of UGTs: neonates have low UGT activity.
-
This abnormality underlies the risk of chloramphenicol toxicity in infants (Gray Baby Syndrome)
-
Infants cannot glucuronidate chloramphenicol efficiently due to immature UGT enzymes, leading to drug accumulation, circulatory collapse, and a characteristic ashen-gray cyanosis.7
-
-
For the same reason, neonates also get physiologic jaundice from inadequate bilirubin glucuronidation.
-
There are also pharmacogenetic differences in UGT capacity.
-
UGT1A1 28 allele (common in Gilbert’s syndrome) results in reduced enzyme expression.8
-
Patients with this variant have mild chronic unconjugated hyperbilirubinemia and may be more susceptible to drugs that require UGT1A1 for clearance.
-
The anti-cancer drug irinotecan is inactivated by UGT1A1; individuals with UGT1A128 (Gilbert variant) are at higher risk for irinotecan-induced neutropenia and diarrhea due to impaired clearance of its active metabolite.9
-
-
-
-
-
The HIV protease inhibitor atazanavir can cause significant hyperbilirubinemia in UGT1A1*28 carriers by competitively inhibiting bilirubin glucuronidation.10
-
-
-
-
These reactions are catalyzed by sulfotransferases (SULT enzymes) which attaches a sulfate group to substrates, usually to phenolic –OH groups).
-
Specifically, sulfotransferases transfer a sulfonate group (SO3-) from a co-substrate, 3'-phosphoadenosine-5'-phosphosulfate (PAPS), to the drug's hydroxyl or amino group.
-
In contrast to glucuronidation, sulfation is generally a high-affinity, low-capacity pathway.13
-
This means it is very efficient at metabolizing low concentrations of a drug but becomes easily saturated as the drug dose increases.
-
-
-
Sulfation is an important pathway for some drugs and many endogenous compounds (e.g. steroid hormones, catecholamines, and thyroid hormone).
-
For drugs, acetaminophen is chiefly cleared by glucuronidation and sulfation (together about 90% of a therapeutic dose).11
-
-
Sulfation often plays a role at lower substrate concentrations, whereas glucuronidation takes over at higher concentrations (as the sulfate pool can deplete).
-
While no common clinical genetic syndromes of sulfotransferase deficiency are noted, variability in sulfation capacity can still affect drug levels.
-
An example of a drug requiring sulfation for activation is minoxidil which is activated to minoxidil sulfate by sulfotransferase in hair follicles.
-
This reaction is relevant to its use in androgenetic alopecia.12
-
-
-
-
-
These reactions are catalyzed by N-acetyltransferases (NAT enzymes) which transfers an acetyl group from acetyl-CoA to a drug’s amine or hydrazine group.
-
The most prominent enzyme is NAT2, which shows genetic polymorphism in humans, classifying individuals as “slow acetylators” or “fast acetylators.”14
-
This polymorphism has classic clinical implications.
-
Isoniazid (an anti-tubercular drug) is metabolized by NAT2; slow acetylators acetylate isoniazid at about half the rate of fast acetylators and have higher plasma isoniazid levels for longer.14
-
Slow acetylators are more prone to isoniazid’s dose-dependent toxicity, such as peripheral neuropathy due to isoniazid-induced pyridoxine deficiency and hepatotoxicity.
-
To prevent neuropathy, all patients on isoniazid are given vitamin B6 (pyridoxine), but slow acetylators still require close monitoring.15
-
-
-
-
NAT2 slow acetylators are at higher risk for drug-induced lupus erythematosus caused by drugs like hydralazine and procainamide.16
-
Both of these drugs are acetylated in Phase II; slow acetylation is associated with a buildup of a reactive intermediate and a greater likelihood of triggering lupus-like autoimmune reactions.
-
Clinically, one finds that up to 50% of Caucasians and African-Americans are slow acetylators, whereas a higher proportion of East Asians are fast acetylators. Pharmacogenetic testing for NAT2 is not routine, but these population differences explain some variability in drug responses.
-
-
Other drugs metabolized by NAT2 include sulfonamide antibiotics, procainamide, and dapsone and slow acetylation of these agents are associated with higher incidence of adverse effects (e.g. sulfonamide hypersensitivity).
-
-
-
-
Methylation reactions are catalyzed by methyltransferases, using S-adenosylmethionine as methyl donor, and adding a methyl group to drugs or endogenous substrates.
-
Methylation generally decreases drug polarity, contrasting with other Phase II reactions.
-
Methylation is a key pathway for catecholamine neurotransmitters (COMT methylates epinephrine, norepinephrine) and for certain drugs.
-
One example is thiopurine methyltransferase (TPMT), which methylates and inactivates thiopurine drugs such as azathioprine, 6-mercaptopurine, 6-thioguanine.17
-
Thiopurine methyltransferase is highly polymorphic given that ~10% of people have intermediate activity and ~0.3% have virtually no TPMT activity due to genetic variants.18
-
Patients exhibiting TPMT deficiency, standard doses of azathioprine or 6-MP may experience severe, life-threatening bone marrow suppression because the active thioguanine nucleotide metabolites are not being inactivated.
-
For this reason, FDA recommends considering TPMT genotyping or phenotyping before starting thiopurines, and guidelines advise dose reduction in TPMT heterozygous or deficient individuals.17
-
Azathioprine’s drug label notes that homozygous TPMT-deficient patients generally require alternative therapies or drastic dose reduction to avoid fatal pancytopenia.19
-
-
-
-
Methyltransferase polymorphisms also play a role in neurotransmitter metabolism with catechol-o-methyltransferase (COMT) variants potentially influencing catecholamine levels.
-
To a limited degree these affects may be associated with pain sensitivity and psychiatric drug responses.20
-
-
-
-
-
-
Glutathione conjugation is catalyzed by glutathione-S transferases (GSTs) and involves coupling of the tripeptide glutathione (GSH) to electrophilic drug molecular centers.
-
This process represents a a major detoxification route for reactive metabolites.
-
An important example is the previously mentioned NAPQI from acetaminophen with hepatocellular GST enzymes conjugate NAPQI with glutathione, neutralizing its reactivity so it can be further metabolized to mercapturic acid and excreted.11
-
If glutathione stores are exhausted (as in overdose), NAPQI escapes this Phase II detoxifying mechanism causing cell injury.
-
Glutathione conjugation also detoxifies many environmental toxins and is involved in resistance to certain chemotherapy agents.
-
Maintaining adequate nutritional status (for GSH precursors) is important for optimal GST function.
-
-
-
-
Major Phase II Enzymes and Examples Phase II Pathway (Enzyme)
Representative Substrates
Pharmacogenetics/Clinical Notes
Glucuronidation (UGT family) Acetaminophen (→ APAP-glucuronide); Morphine (→ morphine-3-glucuronide and -6-glucuronide); Bilirubin; Irinotecan (active metabolite SN-38 glucuronidation); Lorazepam; Lamotrigine
-
Most common Phase II reaction. Neonates have a low UGT → risk of chloramphenicol "Great Baby Syndrome".
-
Gilbert's syndrome (UGT1A28 variant) → decreased bilirubin clearance and risk of toxicity from drugs like irinotecan (FDA recommends dose reductions in UGT1A128 homozygous for irinotecan).
-
Atazanavir inhibits UGT1A1 → benign jaundice in some patients.
Sulfation (SULT family) Acetaminophen (minor pathway → APAP-sulfate); Methyldopa; Steroids (estrogen, DHEA sulfate); Dopamine, Thyroxine.
-
Sulfotransferases have limited sulfate pool; high drug dose saturate sulfation, shifting metabolism other pathways (as in APAP overdose).
-
Generally, no highly polymorphic SULT variant with clinical affected drug metabolism, although SULT1A1 variants may affect hormone metabolism.
Acetylation (NAT1, NAT2) Isoniazid; Sulfasalazine; Hydralazine; Procainamide; Dapsone; Sulfonamide antibiotics.
-
NAT2 is polymorphic: "slow acetylators" (about 50% of subpopulations) metabolize isoniazid and others more slowly leading to high risk of isoniazid peripheral neuropathy and hepatitis.
-
Slow acetylators also at higher risk of drug-induced lupus with hydralazine/procainamide.
-
Fast acetylators may clear drugs to quickly (subtherapeutic levels).
-
Clinicians may just isoniazid dose duration and slow versus fast acetylators, always giving pyridoxine to prevent neuropathy.
Methylation (TPMT, COMT, others) 6-mercaptopurine, Azathioprine, 6-thioguanine (inactivated by TPMT); Catecholamines (inactivated by COMT); Histamine (N-methyltransferase); Nicotine (CYP2D6 & FMO + methylation
-
TPMT polymorphisms: lower or absent TPMT activity causes accumulation of thiopurine active metabolites leading to severe myelosuppression.
-
Guidelines recommend TPMT genotyping or phenotyping before thiopurine therapy; dose reduction or avoidance of low activity.
-
COMT Val/Met variants influenced dopamine metabolism and have been researched in psychiatry (minor clinical impact).
-
Methylation usually reduces drug activity except in rare cases (e.g. codeine’s O-methylation by COMT is not clinically significant compared to CYP2D6 pathway).
Glutathione Conjugation (GSTs) NAPQI (to APAP-GSH conjugate); Reactive phase I metabolites of cyclophosphamide, acetylbenzene epoxides, etc.; Some chemotherapeutics.
-
Crucial detox pathway for electrophilic/toxic intermediates.
-
Glutathione supply can be rate-limiting – e.g. N-acetylcysteine antidote in acetaminophen OD replenishes GSH.
-
GST polymorphisms (e.g. GSTM1) might modestly increase susceptibility to toxicities or cancers, but generally not used to guide therapy.
-
-
-
-