|
press  above to
begin the lecture above to
begin the lecture
return to Pharmacology Table
of Contents
Table of
Contents
- ANS
Anatomy
- Autonomic and Somatic Innervation
- Autonomic
Reflex Arc
- Autonomic Reflex Arc: First Link
- Sensory
Fiber Neurotransmitter(s)
- Autonomic Nervous System
Neurotransmitters: Summary
- CNS and the Autonomic Nervous System
- Spinal Cord Reflexes
- Hypothalamus and Nucleus tractus
solitarii
- Higher
Centers
- Peripheral ANS Divisions
- Comparison
between Sympathetic & Parasympathetic Systems
- Sympathetic
Nervous System Anatomy
- Diagram Sympathetic System
- Anatomical
Outline
- Paravertebral Ganglia
- Prevertebral Ganglia
- Terminal Ganglia
- Adrenal
Medulla
- Parasympathetic
System Anatomy
- ANS
Neurotransmitter Effector Organs
- Eye
- Heart
- Arterioles
- Systemic
Veins
- Lung
|
- Skin
- Adrenal
Medulla
- Skeletal
Muscle
- Liver
- Posterior
Pituitary
|
- Interactions
between Sympathetic & Parasympathetic Systems
- "Fight
or Flight": Characteristics of the ANS
|
- ANS
Neurotransmission
- Neurotransmitter
Criteria
- Neurotransmission Steps:
- Axonal
Conduction
- Storage
and Release of Neurotransmitter
- Combination
of Neurotransmitter and Post-Junctional
Receptors
- Termination
of Neurotransmitter Action
- Other
Non-electrogenic Functions
- Cholinergic
Neurotransmission
- Transmitter
Synthesis and Degradation
- Acetylcholinesterase
- Acetylcholine:
Storage and Release
- Site
Differences:
- Skeletal
Muscle
- Autonomic
Effectors
- Autonomic
Ganglia
- Blood
vessels
- Signal Transduction: Receptors
- Adrenergic
Transmitters: Biosynthetic Pathways
- Adrenergic
Neurotransmission: Introduction to the
Neurotransmitters
- Catecholamine
Synthesis, Storage, Release and Reuptake
- Enzymes
- Catecholamine
storage
- Regulation
of adrenal medullary
catecholamine levels
- Reuptake
- Metabolic
Transformation
- Indirect-acting
sympathomimetics
- Release
- Adrenergic
Receptor Subtypes
- ß-adrenergic
receptors
- Alpha-adrenergic
receptors
- Catecholamine
Refractoriness
- Other
Autonomic Neurotransmitters
- Co-transmission
- ATP
- VIP
- Neuropeptide
Y family
- Purines
- Nitric
Oxide
(Modulator)
- Predominant
Sympathetic/Parasympathetic Tone
- Baroreceptor
Reflexes
- Pharmacological
Modification of Autonomic Function
- Autonomic
Dysfunction
|
Neurotransmitters and the
Autonomic Nervous System
Neurotransmitter Criteria
To support the idea that a chemical is a
neurotransmitter, several conditions must be satisfied:
- The chemical should be found in
the appropriate anatomical location (e.g.
synaptic terminal)
- Enzymes that are involved in
"transmitter" synthesis should also be
present.
- Where possible (as in autonomic
transmission), recovery of the
"transmitter" in higher quantities
following nerve stimulation than in the absence
of stimulation.*
- Externally applied (e.g.
iontophoretically applied) chemical produces the
same effect as stimulation. For example, the
reversal potential is the same.
- Effects of antagonists influence
the response to externally applied chemical in
the same manner as antagonists modify responses
following nerve stimulation.
* may not be possible in many
instances
|
Return
to Table of Contents
Neurotransmission Steps
Axonal conduction
- Depolarization of the axonal
membrane potential results in an action
potential.
- The upstoke of the action potential
is a sodium current flowing through
voltage-activated sodium channels
- As the membrane potential
decreases, activation occurs of an outgoing
potassium current, which opposes further
depolarization and initiates repolarization.
- Longitudinal spread of local
depolarizing sodium currents results in
progressive, longitudinal activation of sodium
channels and new sites of depolarization. The
rate of conduction is dependent on the number and
synchrony of sodium channel activation.
- Number and synchrony of sodium
channel activation is membrane potential
dependent.
- As the resting membrane
potential decrease (towards 0), fewer
sodium channels will be activated by a
depolarizing influence and conduction
velocity slows.
- In myelinated fibers,
depolarization occurs at the Nodes of Ranvier.
|
Return
to Table of Contents
Synaptic (Junctional) Activity
Storage
and Release of Neurotransmitter
- Small
molecule neurotransmitters (e.g. acetylcholine,
norepinephrine) are synthesized at axonal
terminals and stored in synaptic vesicles
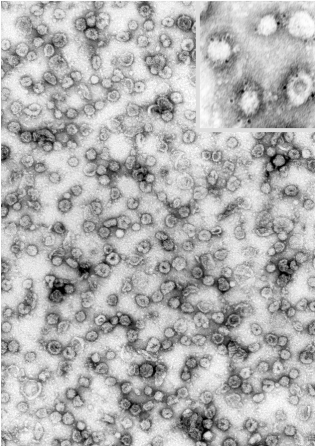
"The electron micrograph shows synaptic vesicles, purified from rat brain (negative staining,
courtesy of Dr. Peter R.
Maycox). Each is about 50 nm in diameter (1/20,000th of a millimeter). The inset shows a
few vesicles labeled by immunogold for one of the major synaptic vesicle proteins
(synaptophysin)."--Research group of Reinhard Jahn (http://www.mpibpc.gwdg.de/abteilungen/190/sv.html)
-
Isolated neurotransmitter
"quanta", perhaps corresponding to
single vesicle neurotransmitter quantity, is
randomly released in the basal state. This level
of release, generating miniature end-plate
potentials (mepp's), is necessary for resting
skeletal muscle tone.
-
Action Potentials, promoting calcium influx, induce
large, synchronous release of several hundred
quanta . Calcium facilitates vesicular
membrane-synaptic membrane fusion, resulting in
vesicular content discharge into the synaptic
cleft.
-
Many chemical can inhibit
norepinephrine or acetylcholine release through
receptor interactions at the appropriate
terminal. Examples:
- Norepinephrine
+ presynaptic alpha 2-adrenergic receptor (autoreceptor)
inhibits norepinephrine release
|
- Alpha2 receptor antagonists increase
release of norepinephrine
|
- Neurally-mediated
acetylcholine release from cholinergic neurons is
inhibited by alpha2-adrenergic receptor agonists
|
- Stimulation
of presynaptic beta2 adrenergic receptors
increases slightly norepinephrine release
|
These agents Inhibit neurally-mediated norepinephrine released by
interacting with presynaptic receptors
| Adenosine |
Acetylcholine |
Dopamine |
Prostaglandins |
Enkephalins |
|
Return
to Table of Contents
Neurotransmitter +
Post-Junctional Receptors Interactions Lead to Physiological Response
- Neurotransmitter
diffuses across the synaptic cleft and bind to
post-junctional receptors causing an increase in
membrane conductance (ions flow)
Three primary types of
changes in conductance may occur:
- increase
in Na+ (usually) or Ca+
conductance which depolarizes the membrane (EPSP)
|
- Increase in Cl-
permeability: inward hyperpolarizing flow
: membrane potential more negative) (IPSP)
|
- Increase
in K+ permeability; K+
leaves the cells, resulting in hyperpolarization, (IPSP)
|
- If the EPSP is of
sufficient magnitude to cause the membrane potential to reach
the threshold potential, an action potential results
(e.g. in skeletal or cardiac muscle). In gland
cells an EPSP may cause secretion; in other
cells, an EPSP may increase the rate of
spontaneous depolarization.
- An IPSP (produced in
neurons and smooth, but not skeletal muscle)
opposes EPSPs.
EPSP: excitatory postsynaptic potential; IPSP:
inhibitory postsynaptic potential
|
Return
to Table of Contents
Termination
of Transmitter Action
- Cholinergic: Termination of action of
acetylcholine is acetylcholine hydrolysis. (acetylcholinesterase-catalazed)
- If acetylcholinesterase is
inhibited, the duration of cholinergic
effect is increased.
- Adrenergic: Termination of action of
adrenergic neurotransmitters is by reuptake and
diffusion away from receptors.
- Amino Acids: Termination of action of
amino-acid neurotransmitters is by active
transport into neurons and glia
|
Return
to Table of Contents
Other
Nonelectrogenic Functions
- Basal, quantal release of
transmitter in quantities insufficient to
generate an EPSP may have other actions. These
effects may include:
- regulation of
neurotransmitter biosynthetic and degradative
enzymes
- pre- and
post-synaptic receptor density
|
Return
to Table of Contents
Cholinergic Neurotransmission
Transmitter
Synthesis and Degradation
- Acetylcholine is synthesized from the
immediate precursors acetyl coenzyme A and
choline in a reaction catalyzed by choline
acetyltransferase (choline acetylase).
|
Return
to Table of Contents
Acetylcholinesterase
- Rapid inactivation of acetylcholine
is mediated by acetylcholinesterase.
- Acetylcholinesterase is present at
ganglia, visceral neuroeffector junctions, and neuromuscular
junctional endplates.
- Another type of cholinesterase,
called pseudo-cholinesterase or
butyrylcholinesterase has limited presence in
neurons, but is present in glia. Most
pseudocholinesterase activity is found in plasma
and liver.
- Pharmacological
effects of anti-cholinesterase drugs are due to
inhibition of acetylcholinesterase.
|
Return
to Table of Contents
Acetylcholine
Storage and Release
- Small random release of
acetylcholine-quanta, producing miniature
end-plate potentials (mepps) , are released by
presynaptic terminals.
- These small currents were
linked to ACh release since
anticholinesterases (neostigmine)
increased their effects, while
cholinergic receptor antagonist
(tubocurarine, a nicotinic receptor
blocker) blocked.
- Anatomical counterpart to the
electrophysiological quanta is the synaptic
vesicle.
- The model is based on the
nicotinic, skeletal neuromusclar junction.
- Synchronous exocytotic release of
many more quanta, dependent on Ca2+
occur when an action potential reaches the
terminal.
- Exocytotic release of
acetylcholine and other neurotransmitters is
inhibited by toxins elaborated by Clostridium botulinum.
|
Cholinergic
Transmission: Site Differences
Skeletal
Muscle
- Neurotransmitter: Acetylcholine
- Receptor Type: Nicotinic
- Sectioning and
degeneration of motor and post-ganglionic nerve
fibers results in:
- an enhanced post-synaptic
responsiveness, denervation
hypersensitivity.
- Denervation hypersensivity
in skeletal muscle is due to
- increased expression of
nicotinic cholinergic receptors
- and their spread to
regions aways from the endplate.
|
Return
to Table of Contents
Autonomic Effectors
- Neurotransmitter: Acetylcholine
- Receptor type: Muscarinic
- effector coupled to receptor by a G
protein
- In smooth muscle and in
the cardiac conduction system, intrinsic
electrical activity and mechanism activity is
present, modifiable by autonomic tone.
- Activities include
propagated slow waves of depolarization:
Examples: intestinal motility and
spontaneous depolarizations of cardiac SA
nodal pacemakers.
- Acetylcholine
decreases heart rate by decreasing the rate of SA nodal
pacemaker phase 4 depolarization.
|
Autonomic Ganglia
- Neurotransmitter:
Acetylcholine
- Receptor
type: Nicotinic
- Generally similar to skeletal muscle
site: initial depolarization is due to receptor
activation. The receptor is a ligand-gated
channel.
|
Return
to Table of Contents
Blood
vessels
- Choline
ester administration results in blood vessel
dilatation as a result of effects on
prejunctional inhibitory synapses of sympathetic
fibers and inhibitory cholinergic (non-innervated
receptors).
- In isolated blood vessel preparations,
acetycholine's vasodilator effects are mediated
by activation of muscarinic receptors which cause
release of nitric oxide, which produces
relaxation.
|
Return
to Table of Contents
press the purple
arrow below (right) to go to the next page
|